Problem Set 10
Chem 220
Due : Monday, December 1, 1997
Chapter 13
1. A one hydrogen triplet appears in a 1H NMR spectrum with J = 6.0 Hz. The three peaks appear at d 0.9, 1.0, and 1.1. What is the frequency (MHz) of the spectrometer? (Courtesy of last year's final exam)
2. Compound A, (C4H9Cl) has the following 1H NMR spectrum: d 3.2 (2H, d, J = 7 Hz),
1.9 (1H, multiplet), and 1.0 (6H, d, J = 7 Hz). Deduce the structure of A.
3. Two bottles in a laboratory have the same label, "C5H10Br2". The 1H NMR spectra of
the contents of bottles A and B are as follows: A: d 3.4 (4H, s) and 1.0 (6H, s). B: d 2.4
(4H, quart.) and 1.0 (6H, t). What are the structures of A and B. Explain.
4. An unknown compound A (C8H16) reacts with m-chloroperbenzoic acid to afford B
(C8H16O). When B is treated with H3O+, a meso compound C (C8H18O2) is formed.
Ozonolysis of A followed by dimethyl sulfide reduction gives a single compound D whose
coupled 13C NMR spectrum is: d 208.7 (s), 39 (t), 30 (q), and 10 (q).
What are the structures A-D? Explain your reasoning.
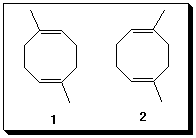
5. A difference of opinion between two of your classmates exists. He insists that the
structures of the constitutional isomers 1 and 2 can be distinguished by 13C NMR
spectroscopy because 2 has a plane of symmetry and 1 does not. He argues that the
number of 13C NMR signals in the decoupled spectrum of each isomer should be different.
She says he's wrong. She suggests a chemical procedure, which was learned in class, to
distinguish between the two compounds. She is correct. Briefly explain his faulty
reasoning and her chemical solution to the problem. (Courtesy of last year's final exam)

6. Two compounds, A and B (C4H10O), are separated by fractional distillation. A has the
following 1H NMR spectra: d 2.4 (1H, t), 3.4 (2H, t), 1.75 (1H, multiplet), and 0.9 (6H,
d); 13C d 69 (s), 30 (s), and 19 (s). When the 1H NMR sample of A is shaken with D2O,
the signal at d 2.4 disappears and the signal at d 3.4 becomes a doublet. For compound B:
1H NMR d 2.9 (1H, t), 3.6 (2H, multiplet), 1.6 (2H, pentuplet), 1.4 (2H, sextuplet), and
0.9 (3H, t). B also exchanges with D2O. What changes are expected? The decoupled
13 C spectrum of B shows four singlets. Show how the structures of A and B can be
deduced from these data?
7. What is the structure of compound A (C6H12O2) shown below? peak areas 3:1.
8. What is the structure of compound A (C4H6Cl2O2) shown below? peak areas ( d 1.0 - 6.0,
3:2:1)