Problem Set 9
Chapters 10 and 11, Alcohols
Due: Monday, April 22, 2013
Solution Set
The alcohol module in ORGO will give you a good review of some of the fundamental reactions discussed in class and in Chapters 10 and 11. As you master the chemistry of alcohols, you should try the Web of Reactions.
1. A Chem220 student needs to prepare 1.5 moles of cyclopentanone from cyclopentanol. Rather than employ heavy metal oxidants that may adversely affect the waste stream, she decides to go GREEN. She employs the method of Stevens, et al., using commercial Clorox® as opposed to "swimming pool bleach". She uses the experimental section of the Stevens' paper as a guide to the stoichiometry of the reaction.
a) Should she use the oxidation of borneol or cyclohexanol as a model experiment? The product of the oxidation of borneol, i.e., camphor, is a solid. It is filtered. Cyclohexanone and cyclopentanone are both liquids, purified by distillation. Go with cyclohexanol!
b) What is the concentration of commercial Clorox®? You can Google this or read it on the label of the container. 6.2% aqueous sodium hypochlorite. I.e., 6.2 g/100 mL.
c) How many gallons of Clorox® will she need? The authors use 1.2 moles of NaOCl for 1 mole of alcohol (20% excess). Therefore, 1.5 moles (129 g) of cyclopentanol requires 1.8 moles of NaOCl. [x = 1.2 x (1.5/1)]. Clorox® has a NaOCl concentration of 0.062 g/mL. Thus, a liter has 62 g/L or 0.83 moles/L. (0.83M; MW NaOCl = 74.5 g/mol). She will need 1.5 mol/0.83 mol/L = 1.8 L of bleach, which is 0.48 US gallons. Better get a full gallon to be on the safe side!

Victor Grignard (1871-1935)
Co-Nobel Prize in Chemistry (1912)
d) How many milliliters of acetic acid will she need? Acetic acid (MW = 60) has a density of 1.05 g/mL, call it 1.0 g/mL. So, 660 mL of acetic acid is ~11 moles. It is a solvent and a reagent. She needs 660 mL x (1.5 mol/1.2 mol) = 825 mL of acetic acid.
2. Optically-active compound A (C10H20O2) reacts with LiAlH4 in ether to form a single optically-active compound B (C5H12O). Bromide C is converted into its Grignard reagent D. Two equivalents of reagent D react with A to form optically-active E (C7H16O) and (R)-B. What are the structures A-E? Explain and illustrate.
A has 1 D.U. It reacts with 2 equivalents of Grignard reagent D. A, with 2 oxygens, is an ester. Since reduction of A with LiAlH4 affords a single optically active B, the alcohol and carboxylic acid portions of the ester must both be C5 and have the same chirality. There is only one possibility for A given that B is of the R-configuration. E is C7 (0 D.U.). Since it is a double Grignard addition of D, D must be methyl magnesium bromide (C5 + C1 + C1 = C7). C is methyl bromide.
3. Identify the unknown compounds in the following series of reactions. Justify your answers.
a) Hydroboration/oxidation gives anti-Markovnikov addition of water to the double bond to give 1-pentanol A after oxidation. PCC (under anhydrous conditions) allows for oxidation to aldehyde D without over oxidation to the carboxylic acid. HBr in the presence of peroxide adds HBr in an anti-Markovnikov fashion affording 1-bromopentane B. C is n-pentyl magnesium bromide. Addition of C to aldehyde D provides 5-decanol E, which is oxidized by hypochlorous acid to 5-decanone F.
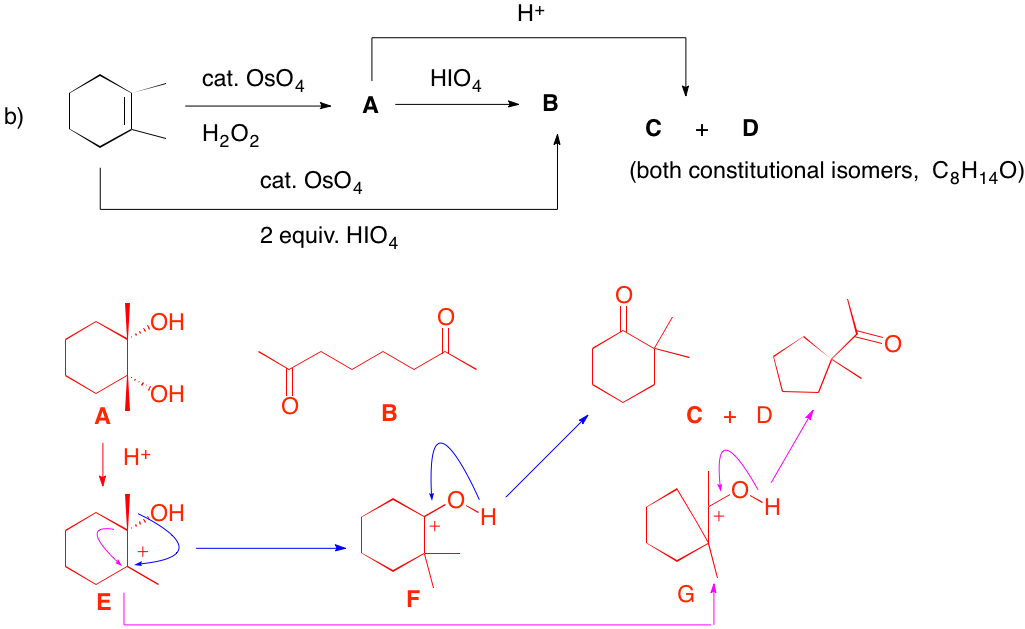
b) OsO4 (VIII) provides cis-dihydroxylation of the double bond to afford diol A. Hydrogen peroxide, as the stoichiometric reagent, reoxides osmium(VI). Periodic acid oxidatively cleaves the diol to diketone B. If 2 equivalents of HIO4 are used as the stoichiometric reagent, A is formed and subsequently oxidized to B. This latter procedure is the operational equivalent of ozonolysis of an alkene followed by dimethyl sulfide reduction. Treatment of the diol A with acid forms the tertiary carbocation E. Migration of the methyl group (blue arrows) forms a new tertiary cation which is more stable than the one from which it was formed because of resonance stabilization from the oxygen atom. F is nothing more than the protonated form of 2,2-dimethylcyclohexanone (C or D). Alternatively, carbocation E can migrate not the methyl group but rather the ring carbon bond (magenta arrows) with its pair of electrons. This process leads to ring contraction to a five-membered ring -- namely, cation G. Deprotonation of G affords 1-(1-methylcyclopentyl) methyl ketone [IUPAC: 1-(1-methylcyclopentyl) ethanone](C or D). C and D are not distinguishable from one another with the information given.
c) Find the structure of oleic acid. LiAlH4 converts the carboxylic acid group to an alcohol affording (Z)-octadec-9-en-1-ol A. Tosylation of A gives tosylate B. Methanolic NaOH is a source of either hydroxide or methoxide anions. Their conjugate acids are approximately six orders of magnitude less acidic than a thiol. [See the pKa tables.] Thus, methanethiol (methyl mercaptan) is converted to its highly nucleophilic anion and effects SN2 displacement of the tosylate group giving sulfide C. Ozonolysis of cyclohexene without reduction leads to ozonide D. [Note: This experiment is usual conducted in a non-nucleophilic solvent.] Sulfide C is used to effect reduction of the ozonide. Racemic sulfoxide E is formed along with adipaldehyde F. The four different substituents on the sulfur of sulfoxide E are the electron pair, the oxygen and two different alkyl groups. Unlike amines, sulfoxides do not invert configurations.
d) The C1 hydrogen of 1-butyne is acidic with a pKa = ~25. The Grignard reagent (alkane pKa = ~50) is more basic than the anion of an alkyne. [See the pKa tables.] The butynide anion opens ethylene oxide to give alcohol B, hex-3-yn-1-ol. PCC oxidation affords aldehyde F, hex-3-yn-1-al. Therefore, A must be the methane formed in the acid-base reaction. Na/NH3 reduces internal alkynes to (E)-double bonds: C = hex-3-en-1-ol. Reaction of alcohol C with PBr3 provides bromide D. Grignard reagent E adds to aldehyde F to yield alcohol G [(E)-dodec-9-en-3-yn-6-ol]. Swern oxidation of secondary alcohol G affords ketone H [(E)-dodec-9-en-3-yn-6-one].
4. Two bottles on a shelf have had their labels fall off. Both of the labels read "C5H11Br". A student decides to run some reactions on the contents of bottle A and B to determine the structures of the two compounds. From the flow chart determine the structure of A and B and identify C-F. Show your reasoning. [Hint: Draw all of the structures of C5H11Br. Eliminate non-contenders? Only the major product in the formation of C should be considered. ]
A has 0 DU. It reacts rapidly with water (SN1). A is a tertiary bromide. The minimum requirement for a tertiary bromide must be 2-bromo-2-methylpropane (C4H9Br). This formula is shy -CH2-. Therefore, A is 2-bromo-2-methylbutane. B is 2-methyl-2-butanol. Tertiary alcohols are not oxidized by PCC. KOH causes E2 elimination of A to form C, 2-methyl-2-butene. Hydroboration of C gives anti-Markovnikov addition of water to the alkene. E is 3-methyl-2-butanol. Chromic acid oxidation of E, a secondary alcohol, provides F, 3-methyl-2-butanone. HBr treatment of secondary alcohol E afford hydride migation product A in addition to direct substitution product B. KOH E2 elimnation of B gives C.
5. Neosporol (1), which is shown in two views, was successfully synthesized from racemic ketone 2, whose synthesis is well beyond the scope of this question. The immediate problem was to convert ketodiol 2 into triol 3. [The fact-oid-s have been altered slighted to facilitate the question. (J. Am. Chem. Soc., 1993, 115, 2581) ] When an excess of methyllithium was used to convert the ketone function of 2 into the tertiary alcohol of 3, only ketodiol 2 was recovered upon aqueous workup. A Jmol structure of neosporol is provided. Move the structure around to compare it with the two views of neosporol 1.
a) What is the minimum amount of methyllithium required in this reaction? Explain? Methyllithium reacts with each of the alcohols and the ketone. Three equivalents of methyllithium.
b) What events occurred prior to aqueous work up? [Hint: Generally, organolithium and Grignard reagents undergo addition but they are also the conjugate bases of weak acids.] What was the fate of the ketone group? Rather than undergoing addition to the ketone, the methyllithium acted as a base, abstracting a hydrogen atom adjacent to the ketone forming a ketone enolate. The enolate is stable until it is protonated in the aqueous workup regenerating the ketone.
When methyl magnesium bromide was employed, both 2 and a mixture of the diastereomers of 3 were obtained. Complete conversion of 2 to 3 (5/1 mixture of diastereomeric tertiary alcohols) was effected cleanly with the cerium reagent, CH3CeCl2.
c) Draw the structures of the two diastereomers of 3, i.e., provide stereochemistry in structure 3. See 3a and 3b below from addition of the organometallic reagent to either face of the ketone.

d) Provide conditions and a mechanism for the conversion of 3 to 1. Is it necessary to separate the diastereomers of 3 prior to forming 1? See above. A proton can protonate any of the oxygen atoms of 3a and 3b. The only productive event is protonation of the tertiary alcohol, which leads to a tertiary carbocation. The carbocation is captured by an intramolecular SN1 reaction followed by loss of a proton to form 1. No separation of 3a and 3b is required.