3. a) Using the standard values for the relative reactivity of 1o, 2o and 3o C-H bonds toward free radical chlorination, determine the relative amount of the four possible monochlorides formed from the chlorination of [2.2.1]-bicycloheptane.
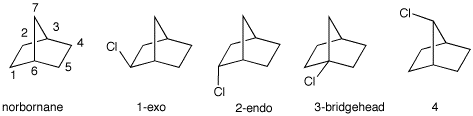
b) The experimental result is: 1, 72%; 2, 25%;
4, 3%; 3, 0%. Why is no bridgehead isomer formed even
though tertiary C-H bonds are highly reactive?
c) There are two types of secondary radicals formed. What is
their relative reactivity? [Hints: Measure the
C2-C1-C6 and the
C3-C7-C6 bond angles in
norborane on the right in which the radical is formed on the
central carbon. How do these values compare with cyclopentane?
The chlorination of cyclobutane is slower than the
chlorination of cyclopentane.]
d) At what point in the radical chain are the endo and exo
isomers formed? Why does the exo isomer dominate? Explain
and illustrate.
e) Are the four monochlorides optically active, racemic, or
achiral?
How to manipulate Jmol structures.