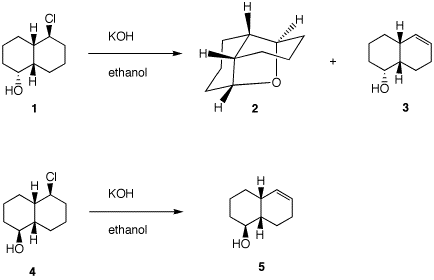
3. A student familiar with the mechanism of the
SN2 and E2 reactions predicts that the
stereoisomer 1 may produce 2 and 3 while stereoisomer 4 will produce only 5 under
the conditions shown. Show her reasoning. [Note: You
need to know how to draw cis-decalins.] [Note: You need to know how to draw cis-decalins.
Look here (.ppt) or here (.html). ]
 |
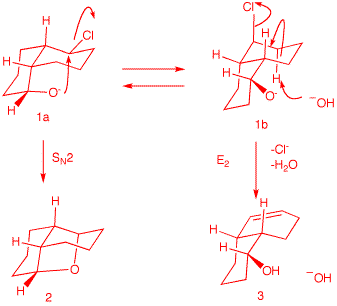
There are two
conformations of cis-decalin 1 --namely, 1a and 1b. Conformation 1a is capable of effecting an
SN2 displacement. Hydroxide reversibly
deprotonates the alcohol, which, in turn, does an
SN2 rear side displacement of chloride.
Use the Jmol structures of 1a and 1b to compare 1a and 1b. Rotate the structures to
get a good view. Click
here.
Conformation 1b has the
alkoxide group remote from the C-Cl bond. The C-Cl
bond is axial and may participate in an E2
elimination in only one direction. There is only
one axial C-H bond anti to the C-Cl bond.
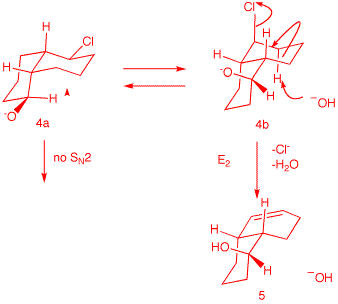
Compound 4 is a diastereomer
of 1, differing only at the
hydroxyl center. Neither conformation 4a nor 4b can effect SN2 displacement. However, E2
elimination from conformation 4b (axial chlorine) is possible. It is likely
that during E2 elimination the alkoxides 1b and 4b are protonated to form the alcohols. |