Problem Set 4
Chapter 5, Stereochemistry
Due: Monday, February 18, 2013
Solution Set
|
Versions of this symbol date to the time of the Vikings. In the 15th century, it was the symbol of a tripartite alliance of the Milanese families Visconti, Sforza and Borromeo via intermarriage. Break any (wedding?) ring and the others separate, hence the alliance is broken. The rings form a chiral object (left) that is not superimposable on its mirror image. A set of Borremean rings has been used as the logo for a certain refreshment that extols purity, body, and flavor. Is the sense of chirality of the two sets of Borremean rings the same or different? For some other discourses on chirality, see: Snails, Snakes and Darwin (html) 1 2 (pdf) 1 2
|
(1824 - 1907) "I call any geometrical figure, or any group of points chiral, and say that it has chirality, if its image in a plane mirror, ideally realized, cannot be brought to coincide with itself." Baltimore Lectures, 1904. |
Read the stereoisomers module in the StudyAids and do the exercises. There is no need to record answers on your homework.
Don't forget the Chirality of Shells (Powerpoint). Do left-handed whelks have a better survival rate than their mirror image brethren? Click here.
|
1. When
(R)-1-chloro-2-methylbutane (1) undergoes free radical
chlorination, five dichloro constitutional isomers are
formed. What are these structures? Draw them. Be explicit as
to diastereomers, enantiomers, racemates, etc. 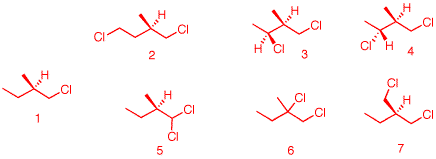
|
|
2. A 5:1 mixture of enantiomers has [α]D = +120o. What is the rotation of the levorotatory enantiomer? The dextrorotatory enantiomer? Show work. Because the net rotation is positive, the major enantiomer must be dextrorotatory; the minor one, levorotatory. A 5:1 mixture is 83.33% (5/6) dextro-, 16.67% (1/6) levo. Thus, +120 = 5/6x(+rot.) + (1/6)x(-rot.); +120 = 2/3x(+rot.); +rot. = 120*3/2 = +180o for the dextrorotatory enantiomer; -180o for the levorotatory enantiomer. |
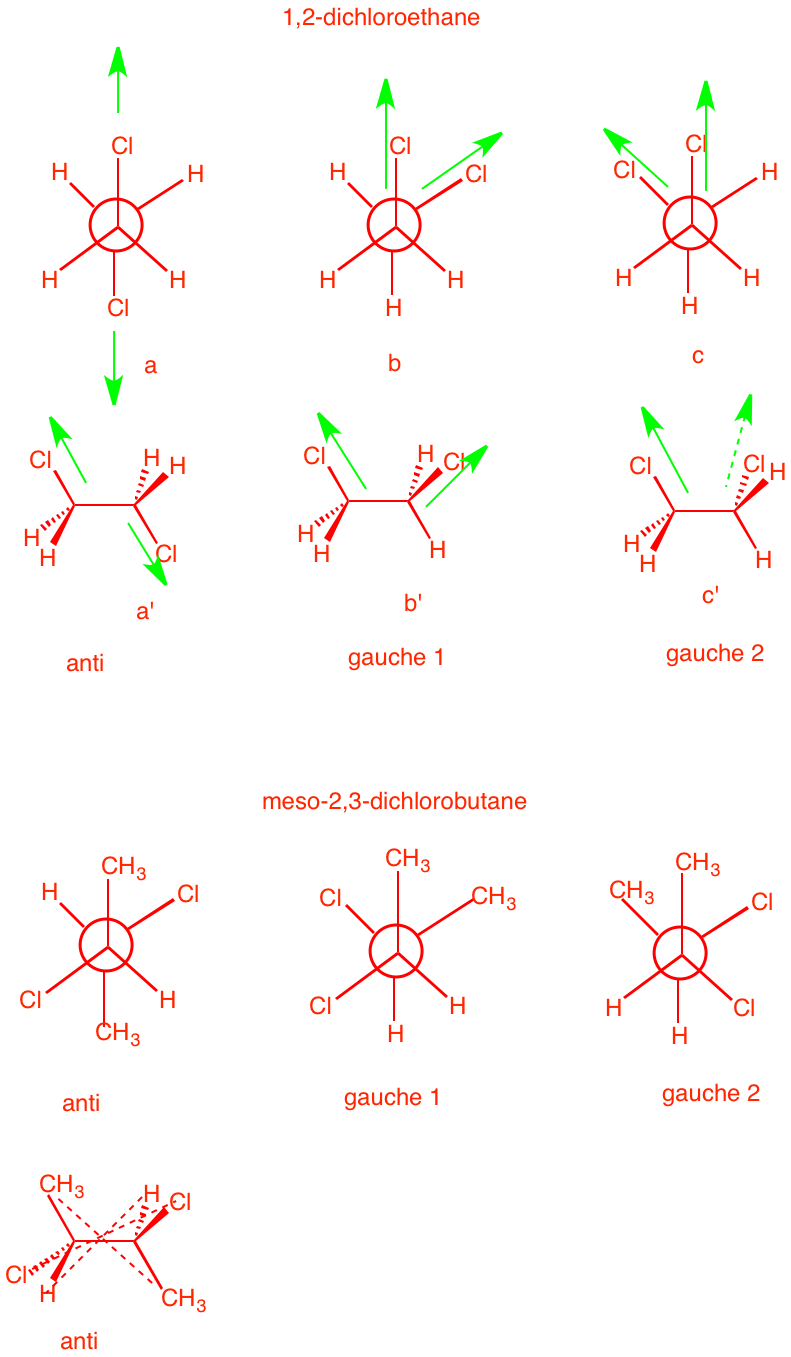
3. a) 1,2-Dichloroethane is optically
inactive yet it has a dipole moment. Explain and illustrate.
[Hint: Draw the staggered conformations and assess
optical activity and dipole moment for each.] Optical activity
is an algebraically additive property; dipole moments are
cumulative in nature. The three staggered conformations of
1,2-dichlorobutane are shown on the right in both Newman
projections and sawhorse views. The anti conformation has no
net dipole. The bond dipoles cancel. [Only the green C-Cl bond dipoles are shown.] The two gauche
conformations have net dipoles. The vector sum of the bond
dipoles gives the molecular dipole. As to optical activity,
the anti conformation is achiral [center of
symmetry]. Gauche 1 and gauche 2 (not to be confused with Dr. Seuss's Thing one and Thing two) conformations are chiral and form
a racemate. No net optical activity. |
|
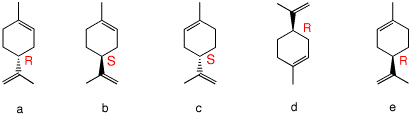
4. Which of the following compounds are, in principle, capable of resolution? Explain and illustrate. [For 3-D JSmol views of these structures click here.: a, b, c, d, e, f, g. ]
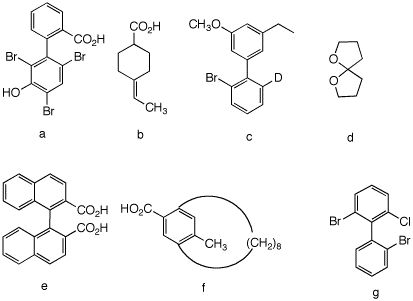
5a) This biphenyl is not planar owing to the three large groups (Br, Br, CO2H) at the ortho positions that inhibit planarity. The two rings are orthogonal to one another thereby producing two non-superimposable mirror images.
b) This compound can be resolved. Imagine that the CO2H group is above the plane of the molecule. Draw its mirror image. They are not superimposable. The double bond and the 6-membered ring are an extension of the cumulated double bonds in the resolvable 1,3-disubstituted allenes.
c) Not resolvable. Free rotation about the biphenyl bond is too rapid for resolution. No different from ortho-bromobiphenyl.
d) Resolvable. Similar to trans-cyclooctene. Not superimposable on its mirror image. This compound is not planar but tetrahedral at the carbon bearing the two oxygens.
e) Resolvable. Same as 5a.
f) Eight CH2 groups in a row are just enough to span the aromatic ring. Neither the CO2H nor the methyl group can pass through the large ring to effect racemization. Cf.; trans-cyclooctene. The compound is resolvable.
g) Resolvable, same as 5a. The ring bearing the single bromine cannot get past the chlorine and bromine on the other ring.
|
5. Terpenes are naturally occurring compounds that are comprised of multiples of the C5 unit isoprene (it looks like 2-methylbutane). Limonene is a monoterpene that occurs as both enantiomers in nature. The (R)-enantiomer has an orange, citrus-like aroma while the (S)-enantiomer has a harsher, lemony fragrance. a) Of the limonenes shown, identify the R
and S
enantiomers. Now, [α]obs = nd([α]d) + (1-nd)([α]l) and [α]obs = nd([α]d) + (1-nd)(-[α]d) gives [α]obs = ([α]d)(2nd- 1) [Note: 2nd - 1 = ee = op] rearranging (([α]obs/[α]d) +1)/2 = nd and nd = ((101.3/123.8) +1)/2 = 0.91 = 9.1% d-limonene and 90.9% l-limonene. |
c) When compounds containing double bonds
are treated with H2 in the presence of a noble
metal catalyst, hydrogen is added to the double bond. In the
case of (R)-limonene, two compounds, A and
B (both C10H20), are
formed. Are they necessarily formed in equal amounts?
Explain. No, they are not necessarily formed in equal amounts. The faces of the trisubstituted double bond are not hydrogenated with equal facility. Think steric hinderance. The
two compounds formed are cis- and
trans-1-isopropyl-4-methylcyclohexane. Both are
achiral. d) The energy difference between the chair conformations of A is greater than the energy difference of the chair conformations in B. What are the structures of A and B? What are the energy differences? Go here for data. See below. Incidently, at 27oC, A has Keq = 610; B has Keq = 1.6. 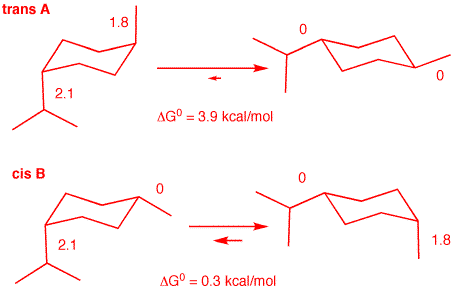
|