Chem 220 - Organic Chemistry Problem Set 7 Chapter 8 - Introduction to Alkyl Halides, Alcohols, etc. Due: October 31, 2011 |
|---|

Potassium cation solvated by the cyclic polyether, 18-crown-6 [18-membered ring; 6 oxygen atoms]. Each of the ethano groups is in a staggered conformation with each of the O-C-C-O dihedral angles at ~60o [gauche]. For a dynamic version, click here. Note that the six oxygen atoms occupy the same spatial arrangement as do the six carbon atoms in chair cyclohexane. The discovery of the crown ethers by Charles Pedersen of DuPont earned him a share in the 1987 Nobel Prize in Chemistry. For potassium ion complexed with a cryptand, click here.
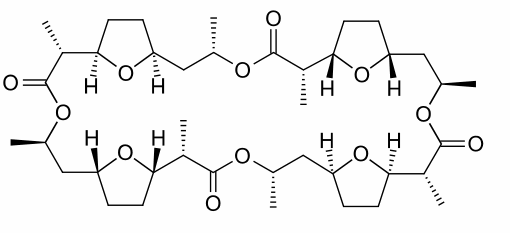
1. Nonactin is a 32-membered ring ionophore comprised of four similar units. Assign R,S - configurations to nonactin and determine whether or not it is optically active. Explain.
Nonactin
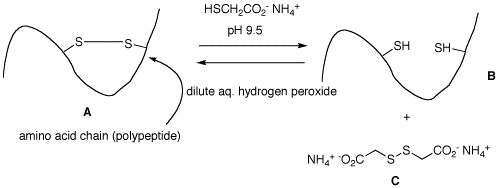
2. Cartoon A represents the cross-linking of a disulfide bond in hair. This property gives hair its natural curl or an artificial "permanent wave". When a solution of ammonium thioglycolate at alkaline pH is applied to the hair, it goes straight to form a dithiol (cartoon B) and disulfide C, which is water soluble and is rinsed away. To restore the curl, the hair is washed with a mild oxidant. Provide a mechanism for the formation of B and C from A, and the formation of A from B.
3. Forty years ago I was a young assistant professor working with a safety expert. We were cleaning up the departmental "chemical morgue". [The morgue was a repository for chemicals that were no longer needed by a researcher and were available to others in the department.] I came across a large bottle of diphenyl ether that was totally solid. Upon informing my associate, he panicked and claimed that the ether bottle (glass, plastic cap) was old; it had totally formed peroxides. As a result, he insisted that we should not handle the bottle but rather call the bomb squad. Your thoughts? How would you test for peroxides? ["Very carefully" is a necessary but not sufficient answer!]
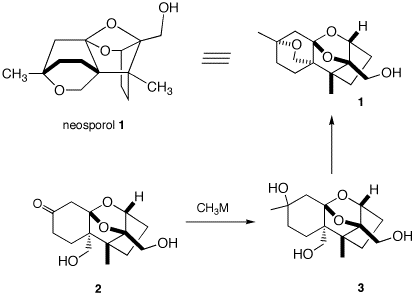
4. Neosporol (1), which is shown in two views, was successfully synthesized from racemic ketone 2, whose synthesis is well beyond the scope of this question. The immediate problem was to convert ketodiol 2 into triol 3. [The fact-oid-s have been altered slighted to facilitate the question. (J. Am. Chem. Soc., 1993, 115, 2581) ] When an excess of methyllithium was used to convert the ketone function of 2 into the tertiary alcohol of 3, only ketodiol 2 was recovered upon aqueous workup. A Jmol structure of neosporol is provided. Move the structure around to compare it with the two views of neosporol 1.
a) What is the minimum amount of methyllithium required in this reaction? Explain?
b) What events occurred prior to aqueous work up? [Hint: Generally, organolithium and Grignard reagents undergo addition to ketones but they are also the conjugate bases of weak acids. pKa's] What was the fate of the ketone group?
When methyl magnesium bromide was employed, both 2 and a mixture of the diastereomers of 3 were obtained. Complete conversion of 2 to 3 (5/1 mixture of diastereomeric tertiary alcohols) was effected cleanly with the cerium reagent, CH3CeCl2.
c) Draw the structures of the two diastereomers of 3, i.e., provide stereochemistry in structure 3.]
d) Provide conditions and a mechanism for the conversion of 3 to 1. Is it necessary to separate the diastereomers of 3 prior to forming 1?
Neosporol