Problem Set 6
Chapter 7: Cyclic Compounds: Stereochemistry of Reactions
Due: Monday, October 24, 2011
The Baeyer Laboratory,
Munich, 1893 (This photograph is in
the hallway across from 110 SCL) Adolf
von Baeyer (1835-1917); Nobel
Prize 1905. (center, seated with derby), who was a student
of Kekulé, succeeded Liebig at Munich. In the
photograph (second row; third from right) is
Henry Lord
Wheeler (1867-1914); Yale Faculty
1896-1911. As was the custom in the 19th century,
many Americans, such as Wheeler, did advanced study in
chemistry in Europe.
Karl
is the laboratory assistant. (The only person wearing an
apron and no tie; upper left.) In 1885, as an addendum to a paper on
acetylenic compounds, Baeyer proposed that cyclopentane was
the least
strained of the cycloalkanes
.
While he accepted the idea that the carbon atoms in
cycloalkanes were tetrahedral, he treated the cycloalkanes
as though they were flat. He argued that there is only one
cyclohexane carboxylic acid, not two (axial and equatorial)
as was predicted by a chair cyclohexane. Equatorial
is frequently misspelled. Sir Derek H. R. Barton
(1918-1998) 1969 Nobel
Prize with Odd Hassel for their
work on conformational analysis For a video of Barton talking about
conformational analysis, click
here.
Cyclohexane
in the chair conformation


Reading and Enrichment
Assignments:
a. Work through How to Draw Cyclohexanes (PowerPoint)
b. The Conformation Module in the Study Aids will give you a good overview of the subject of conformation of cycloalkanes.
1. Compound A (MW=140.22), a 1,4-disubstituted cyclohexane, has the following composition: C, 77.09% and H, 11.50%. (What else is there?) The difference in conformational energy for the two chair conformations of A is 1.3 kcal/mol. Using the A-value data (Energy Differences Between ..... Cyclohexanes), determine the structure of A. Illustrate and explain. What is the conformational energy difference for the stereoisomer of A, ---namely A'. Explain and illustrate. Show the chair comformations of A and A' with the appropriate equilibrium arrows to illustrate the major and minor conformations. Label each conformation with its energy.
2. Unknown compound (R)-(+)-A (C10H16; [α]d=124o) reacts with two molar equivalents of hydrogen to produce two 1,4-disubstituted cyclohexanes, B and C, both of which are optically inactive. Compound B has an energy difference between its two chair conformations of 0.3 kcal/mol, while compound C's difference is 3.9 kcal/mol. Ozonolysis of A provides one mole of formaldehyde and one mole of an optically active diketoaldehyde D. What are the structures of A-D? Show your reasoning.
3. Draw the 3-D line-angle structures of the major products in each of the following reactions. Pay attention to stereochemistry, optical activity and racemic mixtures. Explain briefly.
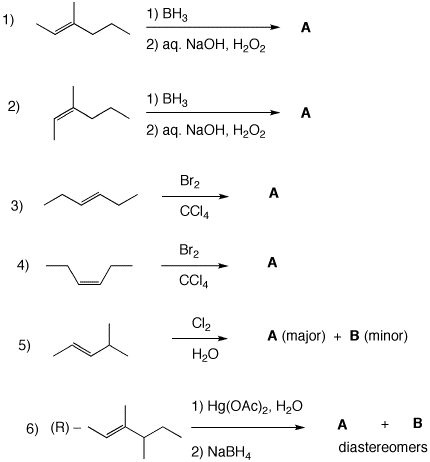
4. In 1886, Albert Ladenburg, synthesized the Socratic poison, coniine [2-propylpiperidine (1)], in racemic form. He resolved the racemate into its enantiomers using the reverse of the technique employed by Pasteur ~25 years earlier. a) What did Ladenburg do? b) Was he able to predict which enantiomer he would isolate in his very first experiment? Elaborate. c) The enantiomer of coniine present in hemlock (Conium maculatum L., Umbelliferae ) is (S)-(-)-coniine, [α]D = -18o. Draw the (S)-enantiomer of coniine. d) Assume that Ladenburg obtained a sample of coniine on his first resolution that had [α]D = +16o. What should he have concluded about his resolving agent? How much of each enantiomer would have been in his sample? |
 |
Albert Ladenburg (1842 - 1911) |
|---|
5. Using the Heats of Formation Table list the values for the cycloalkanes in column 2. Which of these cycloalkanes is the least strained? Place its heat of formation in column 3. Compute the hypothetical heats of formation for the remaining alkanes (Remember the magic number in kcal/mol!). Compute the difference in heats of formation of each cycloalkane (pay attention to + and - signs) in column 4 (column 2 minus column 3). What have you calculated in column 4? Find these data in the Thermochemistry Module and compare your answer. Good or bad correlation?
| Cycloalkane | ΔHfo | ΔHfo for hypothetical unstrained ring | Δ(ΔHfo) |
|---|---|---|---|
| Cyclopropane | |||
| Cyclobutane | |||
| Cyclopentane | |||
| Cyclohexane | |||
| Cycloheptane | |||
| Cyclooctane |
.