Chem220 - Organic Chemistry
Problem Set 5
Chapter 6: Stereochemistry
Due: Monday, October 17, 2011
|
Versions of this symbol date to the time of the Vikings. In the 15th century, it was the symbol of a tripartite alliance of the Milanese families Visconti, Sforza and Borromeo via intermarriage. Break any (wedding?) ring and the others separate, hence the alliance is broken. The rings form a chiral object (left) that is not superimposable on its mirror image. A set of Borremean rings has been used as the logo for a certain refreshment that extols purity, body, and flavor. Is the sense of chirality of the two sets of Borremean rings the same or different? For some other discourses on chirality, see:
|
(1824 - 1907) "I call any geometrical figure, or any group of points chiral, and say that it has chirality, if its image in a plane mirror, ideally realized, cannot be brought to coincide with itself." Baltimore Lectures, 1904. |
Read the stereoisomers module in the StudyAids and do the exercises. There is no need to record answers on your homework.
Don't forget the Chirality of Shells (Powerpoint). Do left-handed whelks have a better survival rate than their mirror image brethren? Click here.
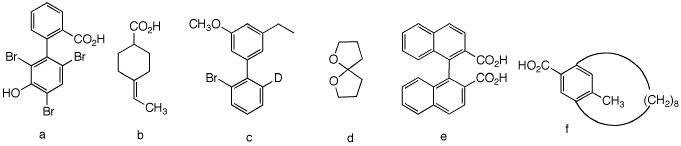
1. Which of the following compounds are, in principle, capable of resolution? Explain and illustrate. [For a JSmol version of this problem. see Spring13, PS4, #4]
2. In the February 23, 2003 Sports Section of the New York Times an article by A. P. Grollman, M.D. appeared on dietary drugs and the effects of ephedra. In the case of Baltimore Oriole pitcher Steve Bechler, 23, the effect was lethal. The active alkaloid in ephedra (ma huang) is ephedrine, (1R, 2S)-1-phenyl-2-aminomethyl-1-propanol. The picture on the right accompanied the article. (1S, 2S)-Pseudoephedrine, a diastereomer of ephedrine, is a common ingredient in decongestants. (The picture is used for educational purposes only.) a) What is the relationship between ephedrine and pseudoephedrine? b) Provide the absolute configuration and name of the stylized structure on the right. Is there a problem? c) Draw sawhorse structures for ephedrine and pseudoephedrine as the enantiomers discussed above. d) Name the four stereoisomers below. Include their CIP configurations. |
|---|
1 |
2 |
3 |
4 |
|---|
How to manipulate JSmol structures
3. a) Label the following structures of carvone as "d" or "l" given the following piece of information: (S)-carvone has a specific rotation of [α]D = +61.
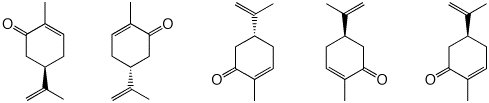
b) A sample of carvone displays n observed rotation of αobs = -40 deg. Which enantiomer is in excess? What percentage of each enantiomer is present? Show your work.
4). The structure and absolute stereochemistry of cholesterol (1) is on the right. a) Excluding the double bond as a source of stereochemistry, how many additional stereoisomers of cholesterol are possible? Explain. b) Label each chiral center using the CIP system. |
1 |
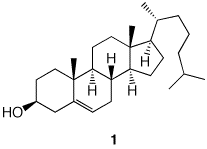 |
|---|
5) When (R)-1-chloro-2-methylbutane undergoes free radical chlorination, five dichloro constitutional isomers are formed. What are these structures? Draw them. Be explicit as to diastereomers, enantiomers, racemates, etc. Which ones are optically active? Which ones are not? Explain.

