Chem220 - Organic Chemistry
Problem Set 5
Solution Set
Chapter 6: Stereochemistry
Due: Monday, October 17, 2011
|
Versions of this symbol date to the time of the Vikings. In the 15th century, it was the symbol of a tripartite alliance of the Milanese families Visconti, Sforza and Borromeo via intermarriage. Break any (wedding?) ring and the others separate, hence the alliance is broken. The rings form a chiral object (left) that is not superimposable on its mirror image. A set of Borremean rings has been used as the logo for a certain refreshment that extols purity, body, and flavor. Is the sense of chirality of the two sets of Borremean rings the same or different? For some other discourses on chirality, see:
|
(1824 - 1907) "I call any geometrical figure, or any group of points chiral, and say that it has chirality, if its image in a plane mirror, ideally realized, cannot be brought to coincide with itself." Baltimore Lectures, 1904. |
Versions of this symbol date to the time of the Vikings. In the 15th century, it was the symbol of a tripartite alliance of the Milanese families Visconti, Sforza and Borromeo via intermarriage. Break any (wedding?) ring and the others separate, hence the alliance is broken. The rings form a chiral object (left) that is not superimposable on its mirror image. A set of Borremean rings has been used as the logo for a certain refreshment that extols purity, body, and flavor. Is the sense of chirality of the two sets of Borremean rings the same or different? For some other discourses on chirality, see:
|

|
Read the stereoisomers module in the StudyAids and do the exercises. There is no need to record answers on your homework.
Don't forget the Chirality of Shells (Powerpoint). Do left-handed whelks have a better survival rate than their mirror image brethren? Click here.
1. Which of the following compounds are, in principle, capable of resolution? Explain and illustrate. [For 3-D Jmol views of these structures click here: a, b, c, d, e, f.]
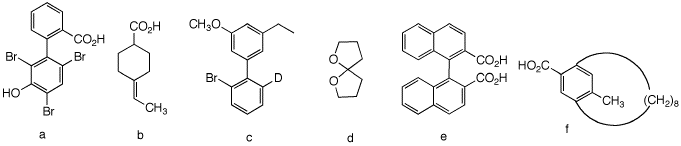
5a) This biphenyl is not planar owing to the three large groups (Br, Br, CO2H) at the ortho positions that inhibit planarity. The two rings are orthogonal to one another thereby producing two non-superimposable mirror images.
b) This compound can be resolved. Imagine that the CO2H group is above the plane of the molecule. Draw its mirror image. They are not superimposable. The double bond and the 6-membered ring are an extension of the cumulated double bonds in the resolvable 1,3-disubstituted allenes.
c) Not resolvable. Free rotation about the biphenyl bond is too rapid for resolution. No different from ortho-bromobiphenyl in terms of free rotation.Of course, ortho-bromobiphenyl has a plane of symmetry.
d) Resolvable. Similar to trans-cyclooctene. Not superimposable on its mirror image. This compound is not planar but tetrahedral at the carbon bearing the two oxygens.
e) Resolvable. Same as 5a.
f) Eight CH2 groups
in a row are just enough to span the aromatic ring. Neither the
CO2H nor the methyl group can pass through the large ring
to effect racemization. Cf.; trans-cyclooctene. The compound is
resolvable.
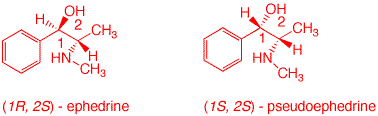
2. In the February 23, 2003 Sports Section of the New York Times an article by A. P. Grollman, M.D. appeared on dietary drugs and the effects of ephedra. In the case of Baltimore Oriole pitcher Steve Bechler, 23, the effect was lethal. The active alkaloid in ephedra (ma huang) is ephedrine, (1R, 2S)-1-phenyl-2-aminomethyl-1-propanol. The picture on the right accompanied the article. (1S, 2S)-Pseudoephedrine, a diastereomer of ephedrine, is a common ingredient in decongestants. (The picture is used for educational purposes only.) a) What is the relationship between ephedrine and pseudoephedrine? Since the C1 configurations are mirror images and C2 configurations are the same, they are diastereomers. b) Provide the absolute configuration and name of the stylized structure on the right. Is there a problem? The structure on the right is a diastereomeric stereoisomer of ephedrine, (1R, 2R) - pseudoephedrine. It is the enantiomer of pseudoephedrine noted in the introduction. There is a problem; the structure is wrong. c) Draw sawhorse structures for ephedrine and pseudoephedrine as the enantiomers discussed above.
d) Name the four stereoisomers below. Include their CIP configurations. |
|---|
1 (1R, 2R) - pseudoephedrine |
2 (1S, 2S) - pseudoephedrine |
3 (1R, 2S) - ephedrine |
4 (1S, 2R) - ephedrine |
|---|
3. a) Label the following structures of carvone as "d" or "l" given the following piece of information: (S)-carvone has a specific rotation of [α]D = +61. Since (S)-carvone has a positive rotation, it is "d". Therefore, (R) = "l".
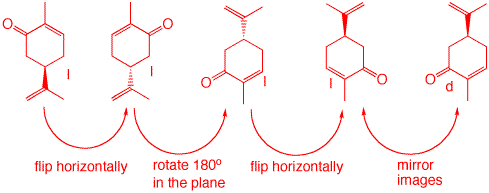
b) A sample of carvone displays an observed rotation of αobs = -40 deg. Which enantiomer is in excess? What percentage of each enantiomer is present? Show your work.
d-Carvone
has a specific rotation of +61o; by definition,
l-carvone is -61o. Let nd =
mole fraction of d-carvone and nl the mole
fraction of l-carvone. The major component is l-carvone because of the net rotation.
Then nd = 1- nl and [α]l= -[α]d
Now, αobs = nl ([α]l) + (nd )([α]d)
and, αobs = nl ([α]l) + (1-nl)([α]d)
αobs = nl([α]l) - (1-nl)([α]l )
gives αobs = ([α]l)(2nl - 1) [Note: 2nl - 1 = ee = op]
rearranging ((αobs/[α]l) +1)/2 = nl
and nl = ((|-40|/61) +1)/2 = 0.828 = 82.8% (R)-(-)-carvone; 17.2% (S)-(+)-carvone.
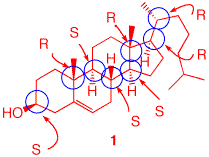
4). The structure and absolute stereochemistry of cholesterol (1) is on the right. a) Excluding the double bond as a source of stereochemistry, how many additional stereoisomers of cholesterol are possible? Explain. The number of stereoisomers is 2n, where n is the number of stereochemical elements, i.e., asymmetric carbons, atropisomers and geometrical isomers (excluded in this case). Cholesterol has 8 asymmetric carbons (blue circles). So 28 - 1 = 256 - 1 = 255 stereoisomers. Not all of them may be possible to exist. b) Label each chiral center using the CIP system. See the structure on the far right. |
1 |
 |
|---|
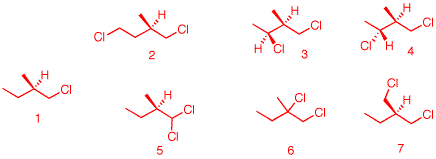
5) When (R)-1-chloro-2-methylbutane undergoes free radical chlorination, five dichloro constitutional isomers are formed. What are these structures? Draw them. Be explicit as to diastereomers, enantiomers, racemates, etc. Which ones are optically active? Which ones are not? Explain. The five constitutional isomers (same molecular formula, different atom connectivity) are: 2, (3, 4), 5, 6 and 7. Still optically active: 2 (R), 3 (2S, 3S), 4 (2S, 3R) and 5 (R). Notice in 3 and 4, which are diastereomers, the (R, S)-configuration has changed at C2 from the monochloro compound although the asymmetric center has not been altered. This is a change in group priority. #6 is racemic because abstraction of the tertiary hydrogen in the first propagation step creates a planar radical. In the second propagation step, chlorination occurs with equal facility on either face of the planar radical. #7 is achiral; two identical groups, -CH2Cl, in the molecule.