
Vladimir Vasilovich
Markovnikov
(1838 -1904)
Chem220 - Organic Chemistry
Problem Set 3
Chapter 4: Alkenes: Structure and Reactivity
Due: Monday, October 3, 2011
Dualism vs. Substitution Theory
The prevailing theory of organic structure in the early 19th century was Dualism or the Electrochemical Theory, principally championed by Berzelius. Since inorganic sodium chloride could be considered as Na+Cl-, then an alkyl halide such as RCl could be thought of as R+Cl-. The R group or "radical" of its day, was thought to be immutable, the carbons and hydrogens behaving as though they were an element. Liebig (German) and Dumas (French), influential chemists of the day, published a joint paper (1837), On the Present State of Organic Chemistry, extolling the concept and claiming all that was left to do in organic chemistry was to identify these immutable radicals (benzoyl, ethyl, acetyl, etc.) As the story goes, a Parisian reception at the Tuileries was to change all of this. [The Tuilerie Gardens was one of the images in Mussorgsky's "Pictures at an an Exhibition", composed in 1874 for piano and later orchestrated by Ravel. The Tuileries selection is here. For the history of this composition and background music, go here.] The guests were discomforted by fumes from the burning candles. Dumas was called in as a consultant. He found that the waxes (fatty esters) had exchanged chlorine for hydrogen, the culprit being the by-product hydrogen chloride. [This story is likely apocryphal. It was told by August Hofmann at a eulogy for Dumas (1884). If the event did occur, it is more likely that the bleaching of candle wax involved addition of chlorine to double bonds. Nonetheless, Dumas did investigate substitution reactions.]
The concept of exchanging electropositive hydrogen for electronegative chlorine was anathema to dualism. Liebig was not enamored with substitution. Why shouldn't he be? After all Liebig and Wöhler had done precisely this in 1832 during their work on the benzoyl radical (C7H5O). They had converted benzaldehyde [(C7H5O)H] into benzoyl chloride [(C7H5O)Cl] by the action of chlorine. So disenchanted was Liebig with the controversies regarding theory in organic chemistry, by 1840 he turned his attention to the practical applications of agricultural chemistry. Thus arose Liebig's beef extract.
Dumas's student, Laurent, not one to shirk from controversy, was bold enough to call the process substitution rather than exchange. Thus, Substitution Theory. Moreover, Dumas (1838) was able to substitute three of the four hydrogens of acetic acid for chlorine to form trichloroacetic acid, which had properties to acetic acid. The recognition of these similar properties led to early Type Theory. In 1842, Melsen, a student of Dumas, reversed Dumas's experiment by reducing trichloroacetic acid to acetic acid by the action of zinc metal. The promulgation of Substitution Theory gave the wry wit of Wöhler, a.k.a., S. C. H. Windler, an opportunity to shine. At the beginning of the 20th century free radicals were detected and named free radicals to distinguish them from the older radicals of Radical Theory of the early 19th century. During the 19th century chemists tried to isolate the older radicals to no avail. When Kolbe and Frankland thought they had isolated methyl, they actually had made the dimer of methyl, ethane. The very process of substituting chlorine for hydrogen is a free radical reaction.
1. In the discussion on Degrees of Unsaturation in your text, the formula U or D.U.= (2C + 2C + 2 + N - H)/2 is employed and incorrect. Eliminate one of the terms "2C". You will notice also that there is no accommodation for halogen, in which case the equation takes the form D.U. = (2C + 2 + N - H - X)/2 or D.U. = C - ((H + X - N)/2) + 1. Derive this formula so that it is yours. most likely you will memorize the formula and get something wrong. Use the alternative method for determining the Degree of Unsaturation (D.U.) shown here. Apply this technique to a substance with the formula C17H22BrClN2O2. Draw a structure that fits this formula.
2. Provide the IUPAC name for the alkene shown here.
3. Using the Heats of Formation table, a) Determine the heat of hydrogenation of (E)- and (Z)-2-hexene. b) What are the heats of formation of (E)- and (Z)-2-decene? Show work. c) Draw a Standard State diagram that relates the heats of formation and hydrogenation of your answers in parts a) and b).
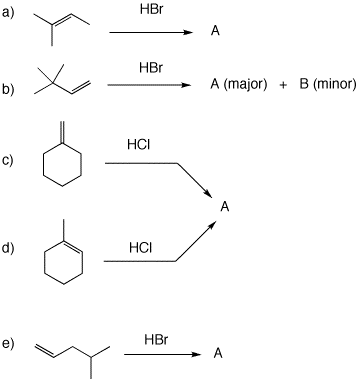
4. Use the curved arrow formalism to show how the structures form in each of the following examples. Pay attention to the mode of Markovnikov addition.
 |
Vladimir Vasilovich Markovnikov (1838 -1904) |
|---|
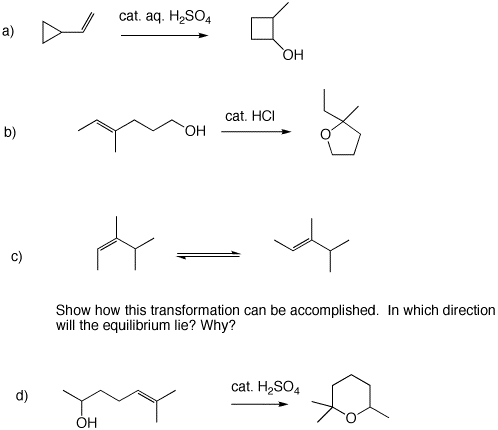
5. Provide mechanisms for each of the following reaction using the curved arrow formalism.