Chem220 - Organic Chemistry
Problem Set 3
Solution Set
Chapter 4: Alkenes: Structure and Reactivity
Due: Monday, October 3, 2011
Dualism vs. Substitution Theory
The prevailing theory of organic structure in the early 19th century was Dualism or the Electrochemical Theory, principally championed by Berzelius. Since inorganic sodium chloride could be considered as Na+Cl-, then an alkyl halide such as RCl could be thought of as R+Cl-. The R group or "radical" of its day, was thought to be immutable, the carbons and hydrogens behaving as though they were an element. Liebig (German) and Dumas (French), influential chemists of the day, published a joint paper (1837), On the Present State of Organic Chemistry, extolling the concept and claiming all that was left to do in organic chemistry was to identify these immutable radicals (benzoyl, ethyl, acetyl, etc.) As the story goes, a Parisian reception at the Tuileries was to change all of this. [The Tuilerie Gardens was one of the images in Mussorgsky's "Pictures at an an Exhibition", composed in 1874 for piano and later orchestrated by Ravel. The Tuileries selection is here. For the history of this composition and background music, go here. (At Yale, try this.] The guests were discomforted by fumes from the burning candles. Dumas was called in as a consultant. He found that the waxes (fatty esters) had exchanged chlorine for hydrogen, the culprit being the by-product hydrogen chloride. [This story is likely apocryphal. It was told by August Hofmann at a eulogy for Dumas (1884). If the event did occur, it is more likely that the bleaching of candle wax involved addition of chlorine to double bonds. Nonetheless, Dumas did investigate substitution reactions.]
The concept of exchanging electropositive hydrogen for electronegative chlorine was anathema to dualism. Liebig was not enamored with substitution. Why shouldn't he be? After all Liebig and Wöhler had done precisely this in 1832 during their work on the benzoyl radical (C7H5O). They had converted benzaldehyde [(C7H5O)H] into benzoyl chloride [(C7H5O)Cl] by the action of chlorine. So disenchanted was Liebig with the controversies regarding theory in organic chemistry, by 1840 he turned his attention to the practical applications of agricultural chemistry. Thus arose Liebig's beef extract.
Dumas's student, Laurent, not one to shirk from controversy, was bold enough to call the process substitution rather than exchange. Thus, Substitution Theory. Moreover, Dumas (1838) was able to substitute three of the four hydrogens of acetic acid for chlorine to form trichloroacetic acid, which had properties to acetic acid. The recognition of these similar properties led to early Type Theory. In 1842, Melsen, a student of Dumas, reversed Dumas's experiment by reducing trichloroacetic acid to acetic acid by the action of zinc metal. The promulgation of Substitution Theory gave the wry wit of Wöhler, a.k.a., S. C. H. Windler, an opportunity to shine. At the beginning of the 20th century free radicals were detected and named free radicals to distinguish them from the older radicals of Radical Theory of the early 19th century. During the 19th century chemists tried to isolate the older radicals to no avail. When Kolbe and Frankland thought they had isolated methyl, they actually had made the dimer of methyl, ethane. The very process of substituting chlorine for hydrogen is a free radical reaction.
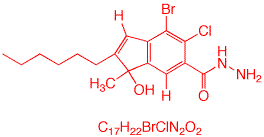
1. In the discussion on Degrees of Unsaturation in your text, the formula U or D.U.= (2C + 2C + 2 + N - H)/2 is employed and incorrect. Eliminate one of the terms "2C". You will notice also that there is no accommodation for halogen, in which case the equation takes the form D.U. = (2C + 2 + N - H - X)/2 or D.U. = C - ((H + X - N)/2) + 1. Derive this formula so that it is yours. most likely you will memorize the formula and get something wrong. Use the alternative method for determining the Degree of Unsaturation (D.U.) shown here. Apply this technique to a substance with the formula C17H22BrClN2O2. Draw a structure that fits this formula.
If you like formulas, let's derive one from what was done above.
The number of hydrogens in the most saturated hydrocarbon must be 2C + 2 less the number hydrogens, halogens and nitrogen equivalents all divided by 2. Since the number of carbons in the most saturated hydrocarbon is increased when nitrogen is replaced by CH, one has D.U. = {[2(C+N)+2]-(H+X+N)}/2. This equation can be reduced to D.U. = C-((H+X-N)/2)+1. For the case at hand, D.U. = 17-(22/2)+1 = 7.
Alternatively,
We begin with C17H22BrClN2O2.
Dropping O gives C17H22BrClN2.
Drop BrCl, add two H: C17H24N2.
Each N is worth CH; therefore, C19H26. [Note: apply other trivalent atoms here, e.g., phosphorus.]
The most saturated C19 hydrocarbon is C19H40. (40-26)/2 = 7 degrees of unsaturation.
| Here is but one of many structures to fit the formula. There are 2 rings and 5 double bonds for a D.U. of 7. |  |
|---|
2. Provide the IUPAC name for the alkene shown here.
| The longest chain containing the double bond is C11; therefore, it is an undecane. Numbering starts on the right with the double bond at C5, not at the sixth carbon from the left. The double bond is (E) owing to the two top priority carbon atoms (C4 and C7) are opposite one another on the double bond. (E)-2-Chloro-5-ethyl-9-methylundec-5-ene (or (E)-2-Chloro-5-ethyl-9-methyl-5-undecene). | 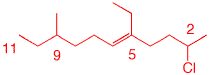 |
|---|
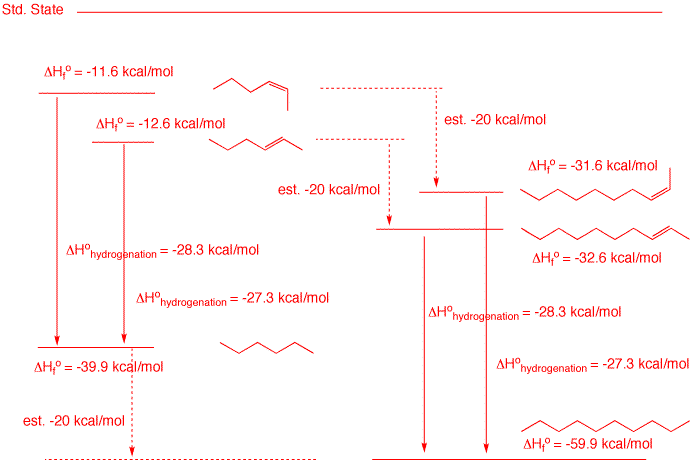
3. Using the Heats of Formation table, a) Determine the heat of hydrogenation of (E)- and (Z)-2-hexene. b) What are the heats of formation of (E)- and (Z)-2-decene? Show work. c) Draw a Standard State diagram that relates the heats of formation and hydrogenation of your answers in parts a) and b).
an examination of the Heats of Formation table reveals that the difference in the heat of formation of normal chain (E)- and (Z)-alkenes is ~1 kcal/mol with the (E)-isomer being the more stable. See 2-butenes, 2-pentenes and 2-hexenes. The latter two alkenes are on the upper left of the diagram.Their product of hydrogenation is n-hexane, whose ΔHfo from the Heats of Formation table is -39.9 kcal/mol. The difference in the heats of formation of the alkenes and alkane gives the heats of hydrogenation. The heats of formation of the C10 compounds are not in the table. Their heats of formation can be estimated by the rule of thumb that an incremental -CH2- = -5 kcal/mol. Since there are four more carbons, -20 kcal/mol can be added to the values for the C6 compounds to obtain the values for the C10 compounds. Obviously, the heats of hydrogenation will remain the same.
4. Use the curved arrow formalism to show how the structures form in each of the following examples. Pay attention to the mode of Markovnikov addition.
a) 2-Methyl-2-butene is protonated at the less substituted end of the double bond (Markovnikov's Rule), which affords the more stable tertiary carbocation (rather than secondary carbocation). Bromide adds to give 2-bromo-2-methylbutane (A).
b) Protonation occurs to give the preferred secondary carbocation to the exclusion of the primary carbocation. Some addition of bromide may occur (green arrows)to form B, but methyl migration is important because a tertiary carbocation is formed. Addition of bromide gives 2-bromo-2,3-dimethylbutane (A).
c, d) Both alkenes protonate to give the same tertiary carbocation, and ultimately 1-bromo-1-methylcyclohexane.
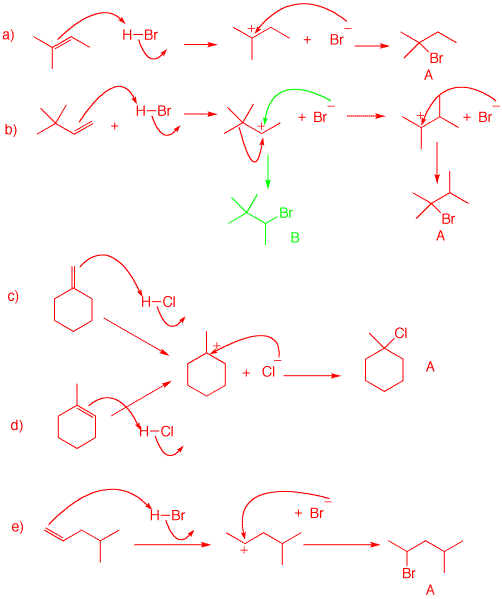
5. Provide mechanisms for each of the following reaction using the curved arrow formalism.
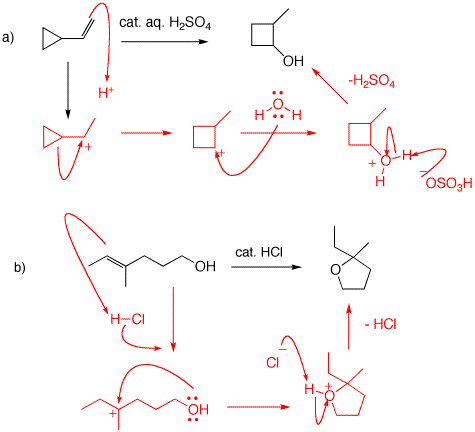
a) The double bond is protonated in the Markovnikov sense to give the secondary (cyclopropyl carbinyl) cation. Hydride migration to give a tertiary cyclopropylmethyl cation does not occur because strain is contained in the 3-membered ring. In stead either, equivalent cyclopropyl carbon bonds migrate to give a secondary cyclobutyl cation. Although the trade-off is secondary for secondary cation, the ring expansion to a 45-membered ring releaves strain. Cation attack by water leads to 2-methylcyclobutanol. Note the catalytic role of sulfuric acid.
b) The double bond is protonated in the Markovnikov sense to give the tertiary cation. The oxygen of the alcohol function acts as a nucleophile, adding to the cation. Intramolecular cyclization occurs. One would not choose water or an alcohol as a solvent in this reaction because, in large excess, they may compete as nucleophiles. Methylene chloride, inert and low boiling point, would be a good choice.
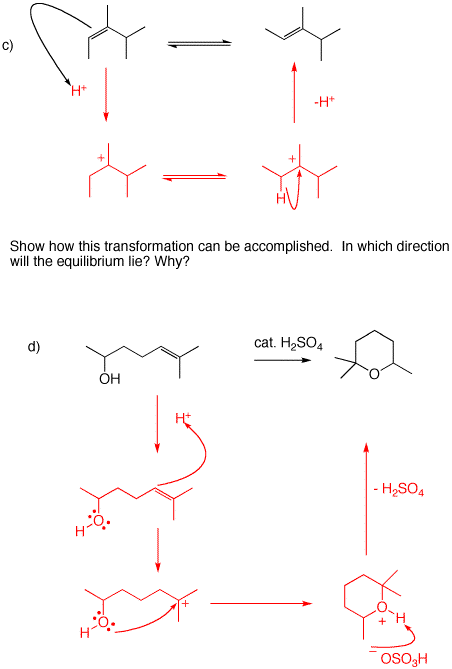
c) Use catalytic acid. The equilibrium will lie to the right because the (E)-isomer is more stable than the (Z)-isomer. That said, the major product of the reaction would be the expected tetrasubstituted alkene, 2,3-dimethyl-2-pentene.
d) The same explanation as 5b.