The alcohol module in ORGO
will give you a good review of some of the fundamental
reactions discussed in class and in Chapters 8 and 9. As
you master the chemistry of alcohols, you should try the
Web
of Reactions. 1. How many grams of KMnO4
in aqueous KOH are required to oxidize 20 grams of
1,2-cyclohexanediol to adipic acid? [Note:
MnO2 is the reduction propuct of permanganate.
This is a redox reaction from Gen. Chem. Go
here for help. Derive the
balanced equation and show your work.]. First, MnO4-
---> MnO2 then add two oxygens
on the right by using two hydroxyls and one water for
each oxygen. 2H2O +
MnO4- ---> MnO2 + 4HO
- (+ 3-electrons; eq. 1) Now, C6H12O2
--->
C6H10O4 requires two oxygen on
the left and two hydrogens on the right. First, the
oxygen: 4HO - +
C6H12O2 --->
C6H10O4 +
2H2O Now the
hydrogens: 2HO - + 4HO
- + C6H12O2
---> C6H10O4 +
2H2O +
2H2O This equation
simplifies to 6HO - +
C6H12O2 --->
C6H10O4 +
4H2O (- 6-electrons; eq. 2) Multiplying eq. 1 by a
factor of two to balance the electrons gained and lost,
we have 4H2O +
2MnO4- ---> 2MnO2 +
8HO - (+ 6-electrons; eq. 3) Adding eqs. 2 and 3,
we have 6HO - +
C6H12O2 +
4H2O + 2MnO4- --->
C6H10O4 +
4H2O + 2MnO2 + 8HO
- Simplifying
yields C6H12O2
+ 2MnO4- --->
C6H10O4 +
MnO2 + 2HO - (eq. 4; Note:
the adipic acid will undergo deprotonation by the
hydroxide at this point. This does not affect the
solution to the problem.) ------------------------------------- KMnO4 MW. =
154; C6H12O2 MW =
116 Thus, 2 x 154/116 =
x/20; x = 54.5 g KMnO4 Victor Grignard
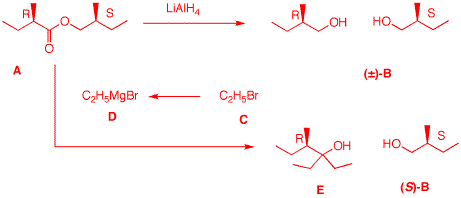
(1871-1935) 2. Optically-active compound A
(C10H20O2) reacts with
LiAlH4 in ether to form a single
optically-inactive compound B
(C5H12O). Bromide C is
converted into its Grignard reagent D. Reagent
D reacts with A to form optically-active
E (C9H20O) and
(S)-B. What are the structures A-E?
Explain and illustrate.
1 DU.
A is C10, 1 DU, two
oxygens, reacts with LiAlH4 and a Grignard
reagent and forms a single C5 compound after
reduction. What is A but an ester
whose carboxylic acid and alcohol portions are both
C5 units. The two fragments of
A must be branched to allow for
optical activity. The racemic alcohol B must be primary
because of the LiAlH4 reduction. There is only
one possibility for a primary alcohol that is
C5 and chiral. At this point the gross
structure of A is known. The lack of
optical activity in B is due to the
presence of a racemate. So the two asymmetric carbons
must have opposite handedness. Esters undergo double
Grignard addition. Since E is
C9 (0 DU), the Grignard reagent
D is ethyl magnesium bromide and
C is ethyl bromide
[C5 + (2 x C2) =
C9]. The production of
(S)-B in the Grignard addition means
that the alcohol portion of ester A
is (S) and the carboxylic acid portion is
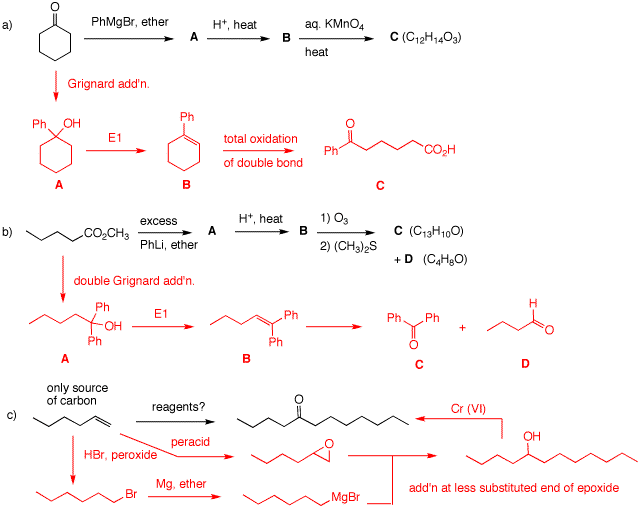
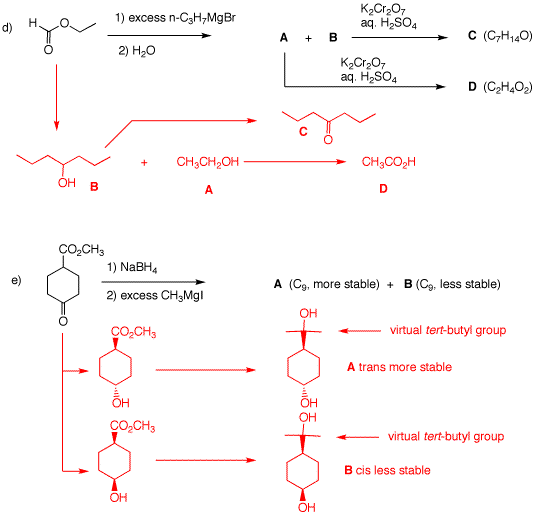
(R). 3. Predict the products and/or
reagents in each of the following examples. Justify your
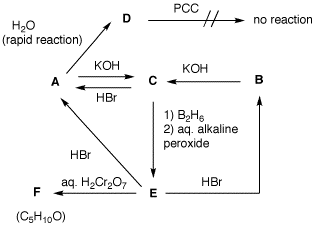
answers. 4. Two bottles on a shelf have had
their labels fall off. Both of the labels read
"C5H11Br". A student decides to run
some reactions on the contents of bottle A and
B to determine the structures of the two
compounds. From the flow chart determine the structure of
A and B and identify C-F. Show your
reasoning. [Hint: Draw all of the structures
of C5H11Br. Eliminate
non-contenders? Only the major product in the formation
of C should be considered.]
A has no
DU. It reacts rapidly with water (SN1).
A is a tertiary bromide. One possibility:
2-bromo-2-methylbutane. B is 2-methyl-2-butanol.
Tertiary alcohols are not oxidized by PCC. KOH causes E2
elimination of A to form C,
2-methyl-2-butene. Hydroboration of C gives
anti-Markovnikov addition of water to the alkene.
E is 3-methyl-2-butanol. Chromic acid oxidation of
E, a secondary alcohol, provides F,
3-methyl-2-butanone. HBr treatment of secondary alcohol
E afford hydride migation product A in
addition to direct substitution product B. KOH E2
elimnation of B gives C.
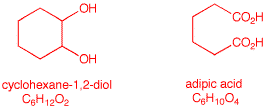
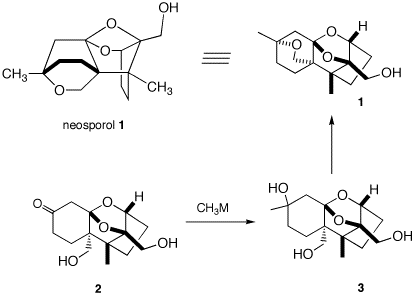
5. Neosporol (1), which is shown in two views, was
successfully synthesized from racemic ketone 2, whose
synthesis is well beyond the scope of this question. The immediate
problem was to convert ketodiol 2 into triol 3.
[The fact-oid-s have been altered slighted to facilitate the
question. (J.
Am. Chem. Soc., 1993, 115,
2581) ] When an excess of
methyllithium was used to convert the ketone function of 2
into the tertiary alcohol of 3, only ketodiol 2 was
recovered upon aqueous workup. A Jmol structure of neosporol is
provided. Move the structure around to compare it with the two
views of neosporol 1.