Problem Set 5
Solution Set
Chapter 6, Alkyl Halides: Substitution and Elimination
Due: Monday, October 11, 2010
xxxxxxxxxxxxxxxxx
Study #2 and #3 in the Alkyl Halide module and #1 in the Ether module
in ORGO.
Problem Set 5
Solution Set
Chapter 6, Alkyl Halides: Substitution and Elimination
Due: Monday, October 11, 2010
xxxxxxxxxxxxxxxxx
Study #2 and #3 in the Alkyl Halide module and #1 in the Ether module
in ORGO.

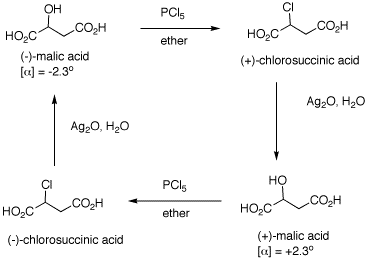
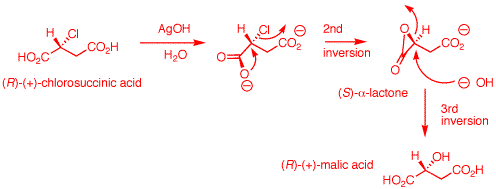
1. The inversion of configuration in an
SN2 reaction is often called a Walden inversion,
named after its discoverer, Paul Walden. In the cycle shown
above, the overall conversion of one enantiomer of malic
acid to the other one must require an inversion of
configuration. Similarly, the same is true of the chloro
acids. More generally, each interconversion of enantiomers
must require an odd number of inversions. The
PCl5 reaction requires a single inversion which
means that the Ag2O reaction involves an even
number of inversions of configuration, namely two in this
instance. (-)-Malic acid is of the
(S)-configuration. a) Show how malic acid, like any alcohol,
might react with PCl5 and then undergo inversion
to form a chloride. Remember that phosphoric acid is a
strong acid and its conjugate base and analogs thereof are
also good leaving groups. b) Silver oxide is an anhydrous form of
AgOH. The carboxylic acid group closest to the hydroxyl
group plays a role in the process. The reaction medium is
mildly alkaline. Using these data, show how there is net
retention of configuration. c) Draw these four enantiomers as Fischer
projections with the CO2H closest to the OH or Cl
in the topmost position. (-)-Malic acid is of the
(S)-configuration.
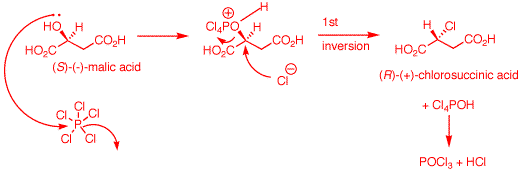
a) An electron pair on
the hydroxyl group of (S)-malic acid does the equivalent of
an SN2 displacement of chloride on the phosphorus
atom of PCl5. The proton at the positive site may
be removed by chloride at this point and then chloride from
dissociated HCl can effect an SN2 displacement.
Alternatively, chloride can effect direct SN2
displacement with inversion of configuration (1st inversion)
to form (R)-chlorosuccinic acid, POCl3 and HCl.
[Note: It is also likely that the
carboxyl groups are converted to acyl chlorides during the
reaction. Aqueous workup would rapidly reform carboxyl
groups]. b) (R)-Chlorosuccinic
acid under alkaline conditions is converted to its
dicarboxylate salt. Ag+1 may or may not complex
with the chlorine atom at this point to enhance chloride as
a leaving group. AgOH is not critical. The reaction works
using NaHCO3 as a base. The proximate carboxylate
acts as a nucleophile with SN2 inversion to form
the reactive, transitory (S)-α-lactone. The strain of
the α-lactone allows hydroxide to effect a second
SN2 displacement to form (R)-malic acid upon
acidification. This step has an even number of inversions --
net retention. The overall process of (R)- to (S)-malic acid
has an odd number of inversions -- net inversion.
c) 2. A sample of (-)-2-iodooctane ([α]D
= -33.3o) reacts with radioactive iodide
(K131I-) in methanol until 1.5%
conversion (i. e.; 1.5% of the isolated 2-iodooctane
contains radioactive iodine.) What is the predicted rotation
of the isolated sample?
The mechanism is
SN2. At 1.5% conversion there is 98.5% of
(-)-2-iodooctane and 1.5% (+)-2-iodooctane. The optical
purity is (98.5 - 1.5)/100 = 97%. Therefore, the expected
rotation is -33.3o x 0.97 =
-32.3o. 3. A student familiar with the mechanism
of the SN2 and E2 reactions predicts that the
stereoisomer 1 may produce 2 and 3
while stereoisomer 4 will produce only 5 under
the conditions shown. Show her
reasoning.
[Note: You need to know how to draw cis-decalins.
Look here
(.ppt) or here
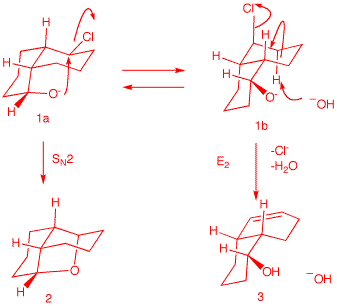
(.html). ] There are two
conformations of cis-decalin 1
--namely, 1a and
1b. Conformation
1a is capable of effecting an
SN2 displacement. Hydroxide reversibly
deprotonates the alcohol, which, in turn, does an
SN2 rear side displacement of chloride.
Use the Jmol structures of 1a
and 1b to compare
1a and
1b. Rotate the structures to
get a good view. Click
here.
Conformation 1b has the
alkoxide group remote from the C-Cl bond. The C-Cl
bond is axial and may participate in an E2
elimination in only one direction. There is only
one axial C-H bond anti to the C-Cl bond.
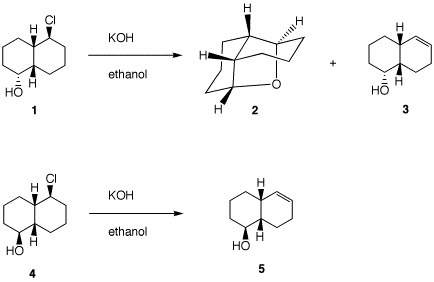
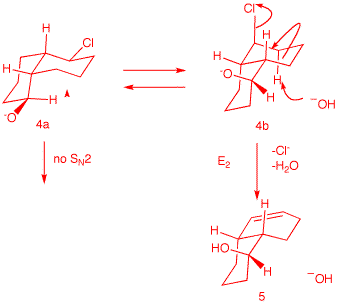
Compound 4 is a diastereomer
of 1, differing only at the
hydroxyl center. Neither conformation
4a nor 4b
can effect SN2 displacement. However, E2
elimination from conformation
4b is possible. It is likely
that during E2 elimination the alkoxides
1b and 4b
are protonated to form the alcohols.
4. Provide the unknown product(s) of each reaction. In all cases,
provide mechanisms and a rationale.
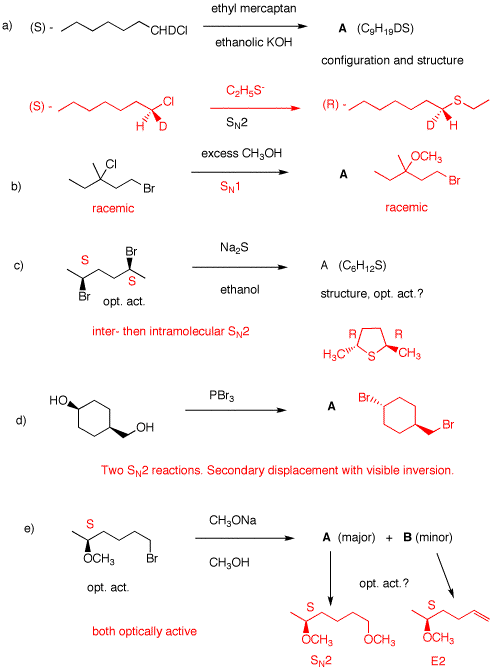
a) Mercaptans (thiols) are
stronger acids than water. See pKa
table. Hydroxide converts
ethanethiol to its thiolate (mercaptide). The anion is a very good
nucleophile. Inversion of configuration occurs. Cl and S are both top
priority.
b) Excess methanol as a solvent. Conditions for an SN1
reaction of the tertiary chloride but not the primary bromide.
Product is also racemic, even if the chloride had been optically
active. Planar carbocation.
c) The dibromide has a two-fold axis of rotation. Both centers are of
the same configuration, ---namely, S. Sulfide (charge -2) does
an intermolecular SN2 dispacement to give an intermediate
that has the new site of the opposite, R-configuration. now,
like 4a, a second SN2 displacement occurs
intramolecularly to give the tetrahydrothiophene A. Both
centers have the R-configuration. A is optically
active.
d) Both hydroxyl groups displace bromide from PBr3 to give
a phosphite (ROPX2). The primary group undergoes
SN2 displacement by bromide. Only the secondary site
affords information about the inversion of configuration, cis --->
trans.
e) The asymmetric center is stable under the reaction conditions. It
remains intact in the products. A small nucleophile and a primary
bromide favors SN2 displacement (Williamson ether
synthesis). A minor amount of E2 product is formed.
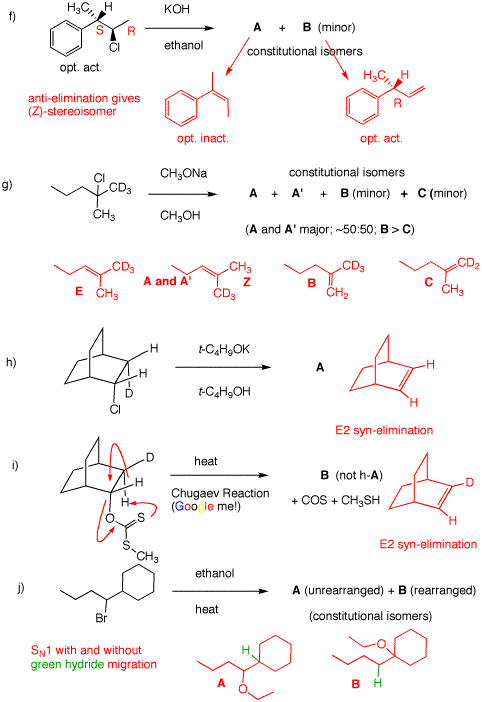
f) Since A and B are constitutional (structural)
isomers and the halide is secondary, elimination will dominate.
A is the more substituted isomer, which suffers E2 elimination
losing HCl in an anti manner. This elimination leads to the Z-isomer
shown. Since both asymmetric centers have become sp2
hybridized, optical activity is lost. The minor E2 product is still
optically active.
g) E2 elimination leads to A and A' having the
trisubstituted double bond. B and C are the minor
1,1-disubstituted double bonds. B dominates over C
because of the isotope effect in E2 eliminations.
h) The D-C-C-Cl dihedral angle is 0o. E2 elimination is
syn overriding any isotope effect. DCl is lost.
i) The Chugaev reaction is an infrequently used procedure for forming
alkenes from alcohols. The alkoxide of the alcohol adds to
CS2 to give a species, RO(C=S)S-, which, after
methyl iodide is added, effects an SN2 displacement to
give a xanthate as shown. Thermolysis gives an intramolecular syn E2
elimination, using the sulfur of the C=S bond as the internal base.
In this case, deuterium remains in the alkene.
j) Ionization of the secondary bromide upon heating can occur to
capture ethanol in an SN1 process. The intermediate
secondary carbocation can have the vicinal
green
hydride migrate to give a more
stable tertiary carbocation, which captures ethanol in an
SN1 process.