|
|
1. In the following problems, provide the missing information. Provide explanations for your choices. Pay attention to stereochemistry.
|
|
2. Cartoon A represents the cross-linking of a disulfide bond in hair. This property gives hair its natural curl or an artificial "permanent wave". When a solution of ammonium thioglycolate at alkaline pH is applied to the hair, it goes straight to form a dithiol (cartoon B) and disulfide C, which is water soluble and is rinsed away. To restore the curl, the hair is washed with a mild oxidant. Provide a mechanism for the formation of B and C from A, and the formation of A from B.
|
|
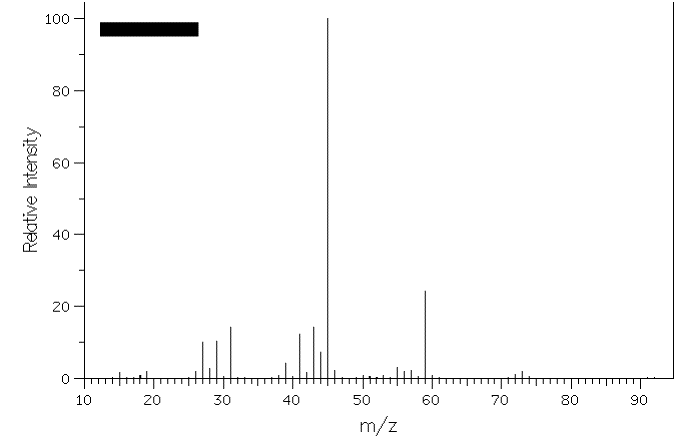
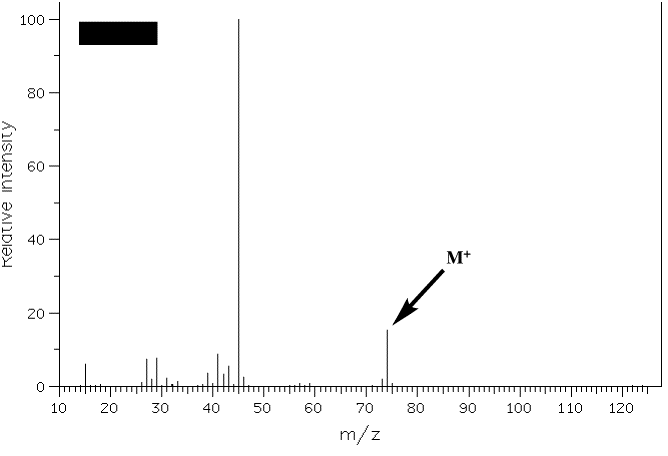
3. The mass spectra of two constitutional isomers, A and B, are shown below. Compound A reacts with pyridinium chlorochromate (PCC); compound B is inert to this reagent. What are the structures of A and B? Interpret the molecular ions and any fragments with m/z greater than or equal to 20% relative intensity. [Note: The mass spectra on pg. 634 (7th ed.) and pg. 632 (6th ed.) will be of help.] Spectrum A: Spectrum B: |
|
4. Design two independent syntheses of 3-cyclopentyl-3-heptanol using two different disconnections and using 1-butene and cyclopentene as the only source of carbon. All other reagents are available to you. What can you say about the optical rotation of 3-cyclopentyl-3-heptanol in each of your syntheses? |
|
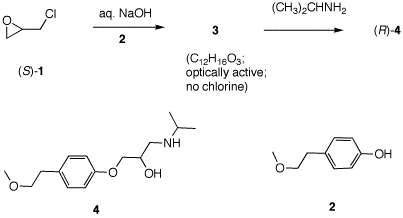
5. Toprol (4; Lopressor; Metoprolol) is a β-blocker used for the treatment of hypertension. It is a racemic drug sold as its monosuccinate salt. A seasoned chemist wants to prepare Toprol as its (R)-enantiomer using the synthesis of the racemate. She follows the steps shown on the right. a) Why is Toprol a racemate? b) Draw a structure of the (±)-succinate salt. c) What is the role of aq. NaOH? Check here. d) What is the structure and CIP designation for 3? e) What does the sequence of reactions and their chirality tell you about the mechanism of the double SN2 reaction sequence? e) Write mechanisms for the two reactions. f) How can she prepare (S)-4 without resorting to the use of (R)-1?
|
 |