|
|
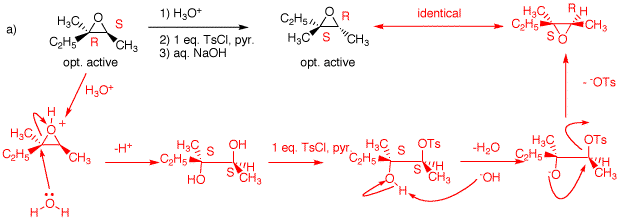
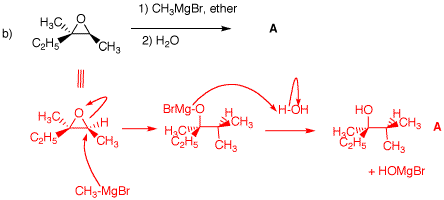
1. In the following problems, provide the missing information. Provide explanations for your choices. Pay attention to stereochemistry. a) The optically-active epoxide is protonated by the acid and opened by SN2 type attack at the more substituted carbon! The more substituted carbon is the site of reaction because in the transition state there is some positive charge on the carbon although most of the positive charge is on oxygen. The more substituted carbon has undergone inversion, R --> S. The secondary alcohol is less hindered than the tertiary one. It is derivatized more readily. Hydroxide forms the alkoxide of the alcohol group, which inverts the stereochemistry at the tosylate carbon (S --> R; intramoleculr Williamson ether synthesis) forming the optically-active epoxide. b) The epoxide in b) is the same as in a), except that it is racemic. Nucleophilic attack is at the the less substituted end of the epoxide. The resulant alkoxide is protonated with water to form (±)-2,3-dimethyl-3-pentanol, A.
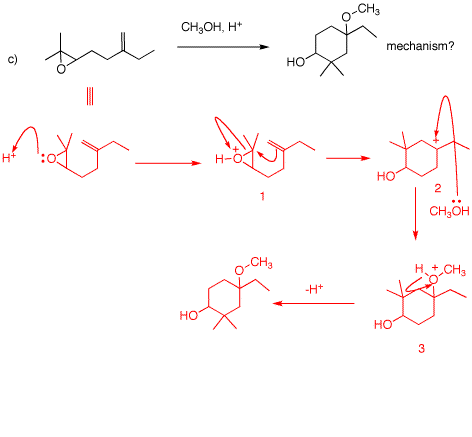
c) The epoxide is protonated. The activated epoxide is attacked at the more substituted end by the weak nucleophilc double such that a tertiary carbocation is formed. The cation reacts with methanol (SN1) to form the product. In spite of the presence of plenty of methanol as a solvent, the proximate alkene reacts preferentially with the epoxide rather than having the epoxide react with the solvent.
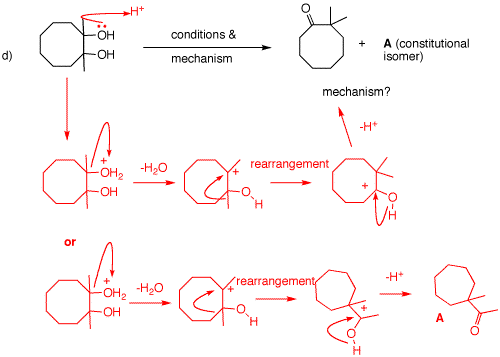
d) A pinacol rearrangement catalyzed by acid. The symmetrical diol, cis or trans, is protonated on oxygen. Dissociation of water occurs, to give a tertiary carbocation. Either a methyl migration occurs to give 2,2-dimethylcyclooctanone or ring contraction occurs to give cycloheptanone A. both pathways occur.
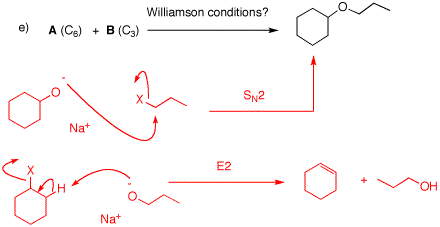
e) The alkoxide of cyclohexanol will give the product efficiently because the SN2 displacement occurs on a 1o carbon. If the alkoxide of 1-propanol is allowed to react with cyclohexyl halide, E2 elimination occurs. X= Cl, Br, I.
|
|
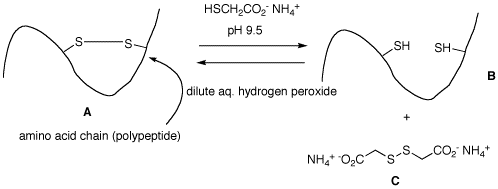
2. Cartoon A represents the
cross-linking of a disulfide bond in hair. This property
gives hair its natural curl or an artificial "permanent
wave". When a solution of ammonium thioglycolate at
alkaline pH is applied to the hair, it goes straight to
form a dithiol (cartoon B) and disulfide C,
which is water soluble and is rinsed away. To restore the
curl, the hair is washed with a mild oxidant. Provide a
mechanism for the formation of B and C from
A, and the formation of A from B.
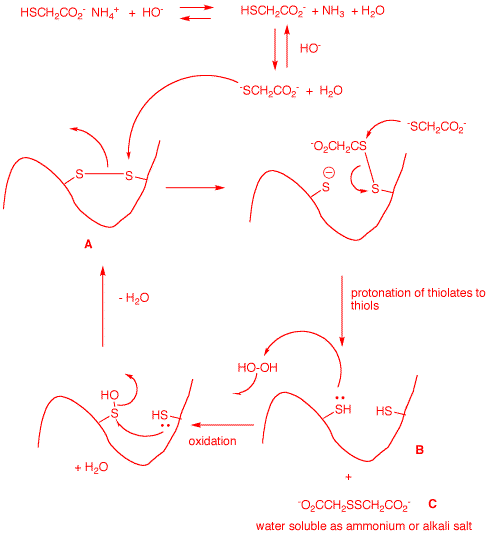
At pH 9.5
ammonium thioglycolate is in equilibrium with ammonia and
the carboxylate and the thiolate carboxylate. It is this
species that behaves as a nucleophile to reduce the
disulfide bonds in hair and form a new, water soluble
disulfide C. Hydrogen peroxide reforms the disulfide
A, putting the "bounce" back.
|
|
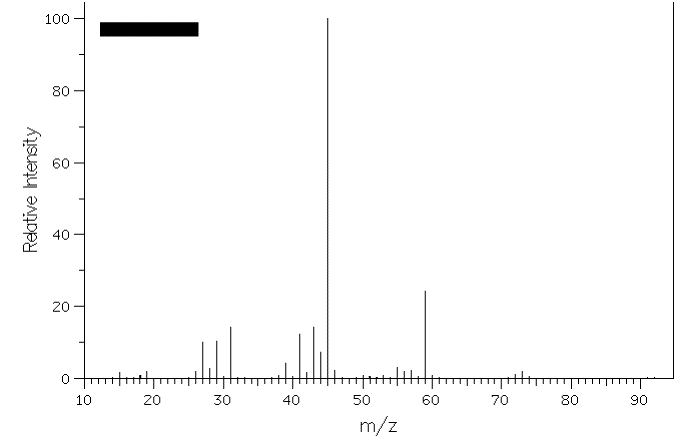
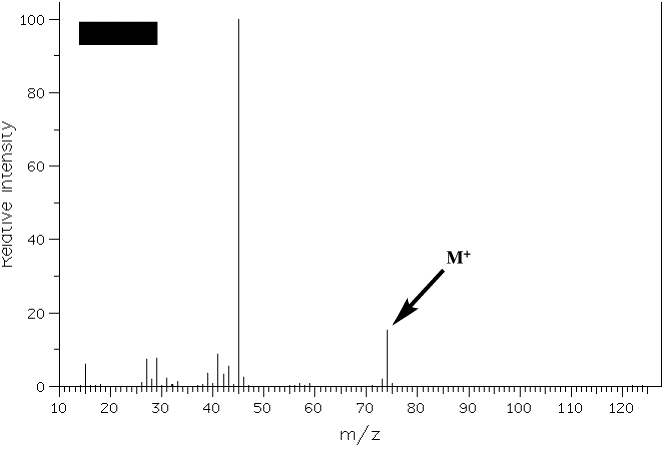
3. The mass spectra of two constitutional isomers, A and B, are shown below. Compound A reacts with pyridinium chlorochromate (PCC); compound B is inert to this reagent. What are the structures of A and B? Interpret the molecular ions and any fragments with m/z greater than or equal to 20% relative intensity. [Note: The mass spectra on pg. 634 (7th ed.) and pg. 632 (6th ed.) will be of help.] Because they are constitutional isomers, both compounds have the same formula, i.e., the same MW 74. One reacts with PCC, therefore each one has at least one oxygen. One is an alcohol and one is an ether or a tertiary alcohol. If it is only one oxygen, then the hydrocarbon portion of the compound is C4H10. There are 4 possible alcohols: 1-butanol, 2-butanol, 2-methyl-2-propanol and 2-methyl-1-propanol. Ethers are: diethyl ether, methyl n-propyl ether and isopropyl methyl ether. 2-Methyl-2-propanol is out because it would show an intense M-15 base peak for loss of a methyl as would isopropyl methyl ether. Diethyl ether is out because its spectrum (in the text) is different from the two shown. The intense base peak (M = 45; M+-29) in spectrum B is due to loss of ethyl from methyl n-propyl ether. Primary alcohols are out because H2C=OH+ (M = 31) would be intense in spectrum A. Therefore, A is 2-butanol with intense peaks at (M = 45; M+-29) loss of ethyl and (M = 59; M+-15) loss of methyl. Spectrum A: Spectrum B: |
|
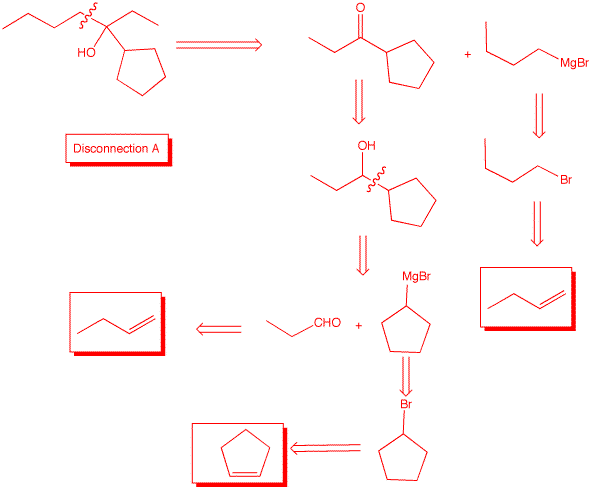
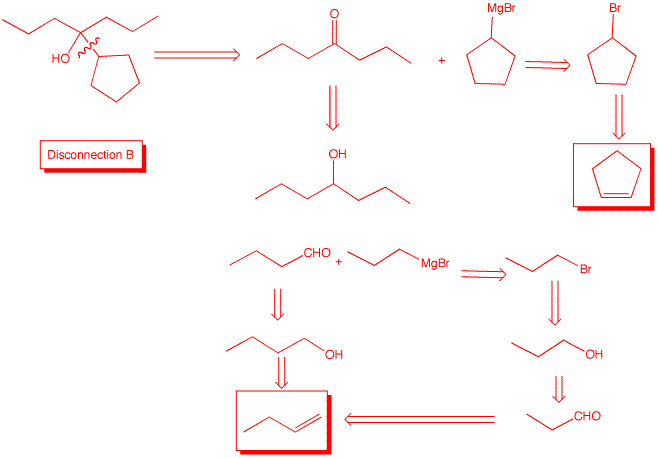
4. Design two independent syntheses of 3-cyclopentyl-3-heptanol using three different disconnections and using 1-butene and cyclopentene. All other reagents are available to you. What can you say about the optical rotation of 3-cyclopentyl-3-heptanol in each of your syntheses?
|
|
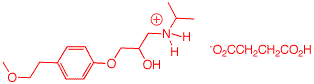
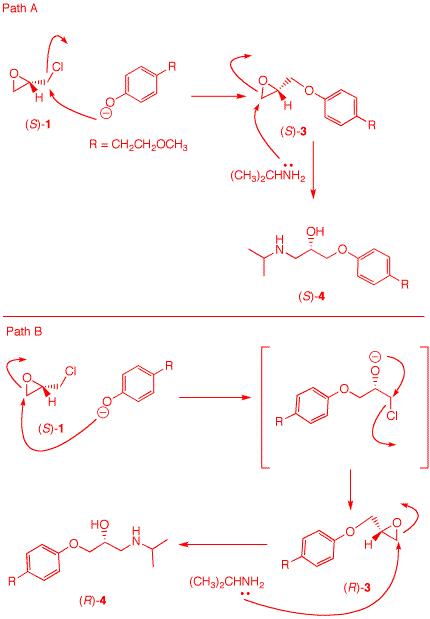
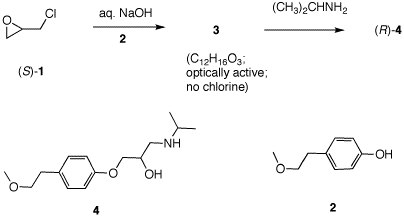
5. Toprol (4; Lopressor; Metoprolol) is a β-blocker for the treatment of hypertension. It is racemic drug sold as its monosuccinate salt. A seasoned chemist wants to prepare Toprol as its (R)-enantiomer using the synthesis of the racemate. She follows the steps shown on the right. a) Why is Toprol a racemate? When prepared from racemic 1, 4 is a racemate because the carbon bearing the OH group is asymmetric. b) Draw a structure of the
(±)-succinate salt. c) What is the role of aq. NaOH?
Check
here.
The strong base
NaOH forms the anion of the phenol 2.
e) What is the structure and CIP designation for 3? See above. f) How can she prepare (S)-4 without resorting to the use of (R)-1? Use the amine as the first nucleophile and the phenoxide second.
|
 |