Due: Monday, November 16,
2009
|
The alcohol module in ORGO
will give you a good review of some of the fundamental
reactions discussed in class and in Chapters 8 and 9. As
you master the chemistry of alcohols, you should try the
Web
of Reactions.
1. How many grams of
K2Cr2O7 in aqueous
H2SO4 are required to oxidize 30
grams of cyclopentanol to cyclopentanone? [This is a
redox reaction from Gen. Chem. Derive the balanced
equation and show your work.] this is a
reduction-oxidaton (redox) reaction.
The alcohol is
oxidized; chromium (IV) is reduced to Cr (III). Both
electrons and atoms must be balanced.
a) balance atoms for chromium:
Cr2O7-2----------->
2Cr+3
Add 7 oxygens on the right as H2O;
Cr2O7-2----------->
2Cr+3 + 7H2O;
Add 14 H+ on the left to balance atoms;
Cr2O7-2 +
14H+ -----------> 2Cr+3 +
7H2O.
b) Balance atoms for cyclopentanol:
C5H10O ------->
C5H8O;
Add 2H+ on the right:
C5H10O ------->
C5H8O + 2H+
c) Determine the net electron change for each half
reaction:
Cr2O7-2 +
14H+ -----------> 2Cr+3 +
7H2O; +12 ---> +6 = +6 electrons;
C5H10O ------->
C5H8O + 2H+; 0 --->
+2 = -2 electrons.
d) Multiply reduction reaction by 3 to balance electron
change..
3 x [C5H10O ------->
C5H8O + 2H+];
3C5H10O ------->
3C5H8O + 6H+;
Add half reactions together:
Cr2O7-2 + 14H+
+ 3C5H10O ----->
2Cr+3 + 7H2O +
3C5H8O + 6H+;
Simplify protons;
Cr2O7-2 + 8H+
+ 3C5H10O ----->
2Cr+3 + 7H2O +
3C5H8O.
The reaction is balanced in atoms and electrons. Three
moles of cyclopentanol are oxidized by one mole of
K2Cr2O7.
MW cyclopentanol = 86; 30/86 = 0.349 moles;
MW K2Cr2O7 = 294; 294 x
(0.349/3) = 34 gm.
|

Victor Grignard
(1871-1935)
Co-Nobel
Prize in Chemistry (1912)
2. Optically-active compound A
(C10H20O2) reacts with
LiAlH4 in ether to form a single
optically-inactive compound B
(C5H12O). Bromide C is
converted into its Grignard reagent D. Reagent
D reacts with A to form optically-active
E (C9H20O) and
(S)-B. What are the structures A-E?
Explain and illustrate.
1 DU.
A is C10, 1DU, two
oxygens, reacts with LiAlH4 and a Grignard
reagent and forms a single C5 compound after
reduction. What is A but an ester
whose carboxylic acid and alcohol portions are both
C5 units. The two fragments of
A must be branched to allow for
optical activity. At this point the gross structure of
A is known. The lack of optical
activity in B is due to the presence
of a racemate. So the two asymmetric carbons must have
opposite handedness. Esters undergo double Grignard
addition. Since E is C9
(0 DU), the Grignard reagent D is
ethyl magnesium bromide and C is
ethyl bromide [C5 + (2 x C2) =
C9]. The production of
(S)-B in the Grignard addition means
that the alcohol portion of ester A
is (S) and the carboxylic acid portion is
(R).

|
|
3. Predict the products in each of the
following examples. Justify your answer.

|
3a)
CH3MgBr adds twice to the ester and once to
the ketone. A stereochemical issue arises because
addition to the ketone can be cis- or trans- to the group
already in the 4-position. A and
B are indistinguishable.
3b) The cis-diol and the trans-diol give the same product
upon aqueous chromium oxidation. The secondary alcohol
affords a ketone while the primary alcohol gives an
aldehyde, which hydrates, and the hydrate is converted to
the carboxylic acid A. For help see
Alcohols #2 in ORGO
here.
3c) The Grignard reagent adds to 3-pentanone to give
3-ethyl-3-pentanol. Acid-catalyzed dehydration can give
but a single alkene B, 3-ethyl-2-pentene.
3d) The rate limiting step is removal of the H or D. It
is harder to break C-D bonds than C-H bonds. With a
limited amount of oxidant, the deuterium-containing
aldehyde A will dominate.
3e) As in 3a stereochemical issues arise. The
LiAlH4 reduction convers both the ester
and the ketone to alcohols. The reduction with
NaBH4 reduces only the ketone.
A and B are
indistinguishable
|
|
4. Two bottles on a shelf have had
their labels fall on the desktop. Both of the labels read
"C5H11Br". A student decides to run
some reactions on the contents of bottle A and
B to determine the structures of the two
compounds. She also has access, as do you, to
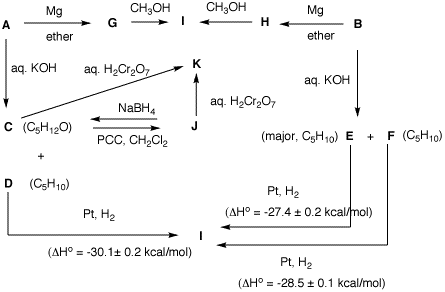
heats
of formation. From the flow
chart determine the structure of A and B
and identify C-I. [Note: The mixture C
and D is derived from A.] Show your
reasoning. [Hint: Draw all of the structures
of C5H11Br. What does I tell
you about A and B?]
There are 8
possible bromides with the formula,
C5H11Br (on the right). The
reactions A -->
G --> I and
B --> H
--> I tell you that
A and B have
the same carbon atom connectivity. Since there is only
bromide arising from the carbon connectivity of 4, 4 is
out of contention. I is a saturated
alkane. The heat liberated in D
--> I says that
D is a terminal alkene formed along
with alcohol C in the base treatment
of A. There are only two options for
D: 1-pentene and 3-methyl-1-butene.
Oxidation of C under aqueous chromic
acid conditions gives K, which is
different from J, which is produced
from C with PCC. Therefore,
K is a carboxylic acid and
J is an aldehyde (convertible to
K). Consequently,
C is a primary alcohol and
A is a primary bromide (1, 5 or 8).
Bromide B gives only alkenes
(E and F) upon
base treatment. B must be (no
alcohol formed) a secondary or tertiary bromide (2, 3, 6
or 7). The difference (~1 kcal/mol) suggests
1,2-disubstituted cis, trans isomers. This info suggests
a normal chain. Since there is no terminal alkene formed
during the elimination of B -->
E and F, 2 is
eliminated. B is 3-bromopentane (3),
E is (E)-2-pentene (less heat
liberated), F is (Z)-2-pentene.
Moreover, A is 1-bromopentane,
C is 1-pentanol,
D is 1-pentene,
I is n-pentane,
J is pentanal and
K is pentanoic acid. No other
secondary or tertiary halides would lead to only two
alkenes whose difference and absolute value of heat of
hydrogenation agrees with the data.
|

|
|
5. Neosporol (1), which is
shown in two views, was successfully synthesized from
racemic ketone 2, whose synthesis is well beyond
the scope of this question. The immediate problem was to
convert ketodiol 2 into triol 3. [The
fact-oid-s have been altered slighted to facilitate the
question. (J. Am. Chem. Soc., 1993,
115, 2581) ] When an excess of methyllithium
was used to convert the ketone function of 2 into
the tertiary alcohol of 3, only ketodiol 2
was recovered upon aqueous workup. A Jmol structure of
neosporol is provided. Move the structure around to
compare it with the two views of neosporol
1.
a) What is the minimum amount of
methyllithium required in this reaction? Explain?
Methyllithium
reacts with each of the acohols and the ketone. Three
equivalents of methyllithium.
b) What events occurred prior to
aqueous work up? [Hint: Generally, organolithium and
Grignard reagents undergo addition but they are also the
conjugate bases of weak acids.] What was the fate of
the ketone group?
Rather than
undergoing addition to the ketone, the methyllithium
acted as a base, abstracting a hydrogen atom adjacent to
the ketone forming a ketone enolate. The enolate is
stable until it is protonated in the aqueous
workup.
When methyl magnesium bromide was
employed, both 2 and a mixture of the
diastereomers of 3 were obtained. Complete
conversion of 2 to 3 (5/1 mixture of
diastereomeric tertiary alcohols) was effected cleanly
with the cerium reagent,
CH3CeCl2.
c) Draw the structures of the two
diastereomers of 3, i.e., provide stereochemistry
in structure 3.
See 3a and
3b below from addition of the organometallic
reagent to either face of the ketone.

d) Provide conditions and a mechanism
for the conversion of 3 to 1. Is it
necessary to separate the diastereomers of 3 prior
to forming 1?
See above. A
proton can protonate any of the oxygen atoms of
3a and 3b. The
only productive event is protonation of the tertiary
alcohol, which leads to a tertiary carbocation. The
carbocation is captured by an intramolecular
SN1 reaction followed by loss of a proton to
form 1. No separation of
3a and 3b is
required.
|





