Problem Set 5
Chapter 6, Alkyl Halides: Substitution and Elimination
Due: Monday, October 12, 2009
xxxxxxxxxxxxxxxxx
Study #2 and #3 in the Alkyl Halide module and #1 in the Ether module
in ORGO.
Problem Set 5
Chapter 6, Alkyl Halides: Substitution and Elimination
Due: Monday, October 12, 2009
xxxxxxxxxxxxxxxxx
Study #2 and #3 in the Alkyl Halide module and #1 in the Ether module
in ORGO.

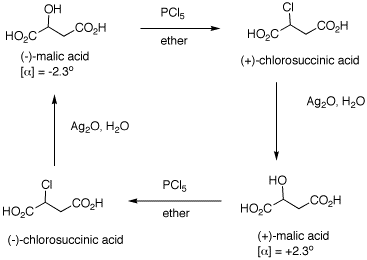
1. The inversion of configuration in an
SN2 reaction is often called a Walden inversion,
named after its discoverer, Paul Walden. In the cycle shown
above, the overall conversion of one enantiomer of malic
acid to the other one must require an inversion of
configuration. Similarly, the same is true of the chloro
acids. More generally, each interconversion of enantiomers
must require an odd number of inversions. The
PCl5 reaction requires a single inversion which
means that the Ag2O reaction involves an even
number of inversions of configuration, namely two in this
instance. (-)-Malic acid is of the
(S)-configuration. a) Show how malic acid, like any alcohol,
might react with PCl5 and then undergo inversion
to form a chloride. Remember that phosphoric acid is a
strong acid and its conjugate base and analogs thereof are
also good leaving groups. b) Silver oxide is an anhydrous form of
AgOH. The carboxylic acid group closest to the hydroxyl
group plays a role in the process. The reaction medium is
mildly alkaline. Using these data, show how there is net
retention of configuration. c) Draw these four enantiomers as Fischer
projections with the CO2H closest to the OH or Cl
in the topmost position. (-)-Malic acid is of the
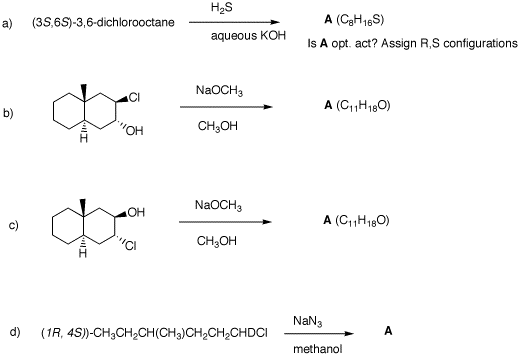
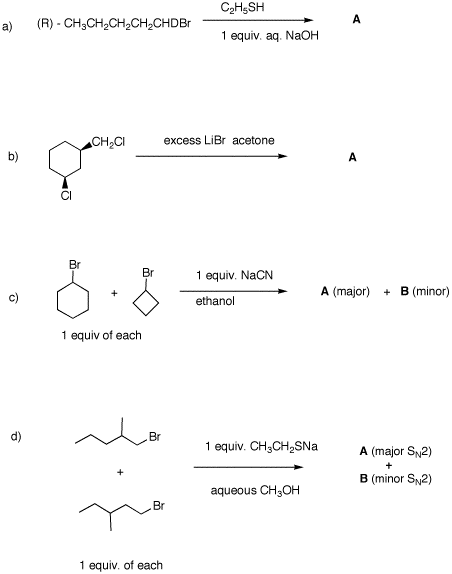
(S)-configuration. 2. In each of the following reactions,
predict the expected products. Explain.
|
3. Show how you would convert (S)-2-octanol into (S)-2-octanethiol. [Hint: how do you make a secondary hydroxyl group a good leaving group for an SN2 reaction?] |