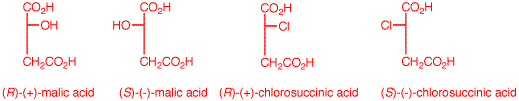Chem 220 - Organic Chemistry
Problem Set 5, Solution
Set
Chapter 6, Alkyl Halides: Substitution and
Elimination
Due: Monday, October 12, 2009
Study #2 and #3 in the Alkyl Halide module and #1 in the Ether module
in ORGO.
|
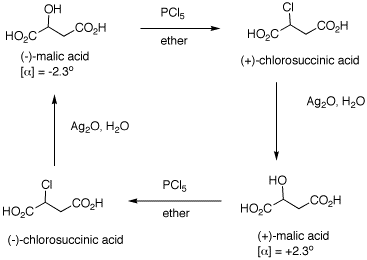
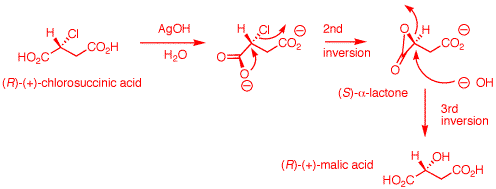
1. The inversion of configuration in an
SN2 reaction is often called a Walden inversion,
named after its discoverer, Paul Walden. In the cycle shown
above, the overall conversion of one enantiomer of malic
acid to the other one must require an inversion of
configuration. Similarly, the same is true of the chloro
acids. More generally, each interconversion of enantiomers
must require an odd number of inversions. The
PCl5 reaction requires a single inversion which
means that the Ag2O reaction involves an even
number of inversions of configuration, namely two in this
instance. (-)-Malic acid is of the
(S)-configuration.
a) Show how malic acid, like any alcohol,
might react with PCl5 and then undergo inversion
to form a chloride. Remember that phosphoric acid is a
strong acid and its conjugate base and analogs thereof are
also good leaving groups.
b) Silver oxide is an anhydrous form of
AgOH. The carboxylic acid group closest to the hydroxyl
group plays a role in the process. The reaction medium is
mildly alkaline.
c) Draw these four enantiomers as Fischer
projections with the CO2H closest to the OH or Cl
in the topmost position. (-)-Malic acid is of the
(S)-configuration.
|
|
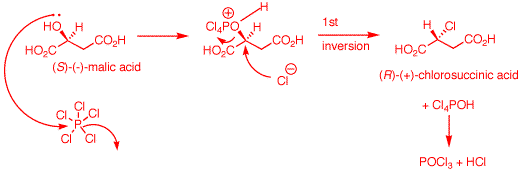
a) An electron pair on
the hydroxyl group of (S)-malic acid does the equivalent of
an SN2 displacement of chloride on the phosphorus
atom of PCl5. The proton at the positive site may
be removed by chloride at this point and then chloride from
dissociated HCl can effect an SN2 displacement.
Alternatively, chloride can effect direct SN2
displacement with inversion of configuration (1st inversion)
to form (R)-chlorosuccinic acid, POCl3 and HCl.
[Note: It is also likely that the
carboxyl groups are converted to acyl chlorides during the
reaction. Aqueous workup would rapidly reform carboxyl
groups].
|
|
|
b) (R)-Chlorosuccinic
acid under alkaline conditions is converted to its
dicarboxylate salt. Ag+1 may or may not complex
with the chlorine atom at this point to enhance chloride as
a leaving group. AgOH is not critical. The reaction works
using NaHCO3 as a base. The proximate carboxylate
acts as a nucleophile with SN2 inversion to form
the reactive, transitory
(R)-a-lactone.
The strain of the a-lactone allows hydroxide to effect a
second SN2 displacement to form (R)-malic acid
upon acidification. This step has an even number of
inversions -- net retention. The overall process of (R)- to
(S)-malic acid has an odd number of inversions -- net
inversion.
|
|
|
c)
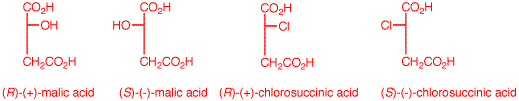
|
|
2.
|
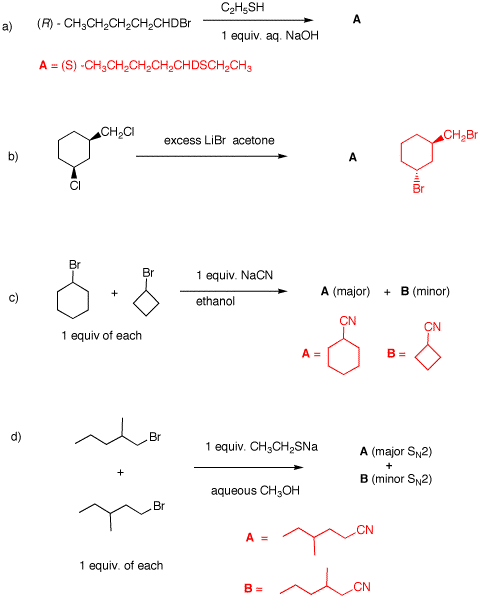
a) This is a primary
bromide that is chiral by virtue of the H, D, Br and n-butyl
group attached to carbon 1. RSH: pKa = 10;
H2O: pKa = 15.7.
LINK TO
PKA
RSH is a weaker acid
than water, so the equilibrium favors RS- +
H2O. Mercaptide ion effects an SN2
inversion to form the (S)-sulfide.
b) Chloride is exchanged for bromide by an SN2
reaction. One can't see the inversion at the primary site
(proved in 2a) but it is seen at the secondary site.
cis-Dichloride to trans-dibromide.
c) An SN2 transition state apNote that there is
approximates a trigonal bipyramid. The apices are the
nucleophile and the leaving group; the trigonal part is
CH2, CH2 and H at ~120o.
The C-C-C bond angle for cyclohexane is 111o;
cyclobutane, 88o. It is easier for cyclohexane to
undergo the displacement as long as elimination to
cyclohexene is not a factor. Note that there is a limited
amount of nucleophile.
d) 1-Bromo-2-methylpentane is more hindered toward
SN2 displacement than 1-bromo-3-methylpentane.
Competition for limited nucleophile favors
1-bromo-3-methylpentane.
|
3. Show how you would convert (S)-2-octanol into
(S)-2-octanethiol. [Hint: how do you make a secondary
hydroxyl group a good leaving group for an SN2
reaction?] a goo
4. (2S,5S)-5-Bromo-2-hexanol (A) is expected to
form optically inactive B (C6H12O) upon
exposure to aqueous NaOH. An optically active stereoisomer of
A, namely, C also forms optically in active B
under the same conditions. Two other optically active stereoisomers
of A, namely D and E as a racemic mixture, form
optically inactive F, a diastereoisomer of B. What are
the structures of A-F? The structures D and E
are not distinguishable. Explain and illustrate with mechanisms. Why
are B and F both optically inactive?