- Chem 220 - Organic
Chemistry
Problem Set 4, Solution
Set
Stereochemistry, Chapter 5
Due: Monday, October
5, 2009

|
The
Borremean Rings
Versions of this symbol date to the time
of the Vikings.
In the 15th century, it was the symbol of a tripartite
alliance of the Milanese families Visconti, Sforza and
Borromeo via intermarriage. Break any (wedding?) ring and
the others separate, hence the alliance is broken. The rings
form a chiral
object (left) that is not
superimposable on its mirror
image. A set of Borremean rings
has been used as the logo for a certain refreshment that
extols purity, body, and flavor. Is the sense of chirality
of the two sets of Borremean rings the same or different?
For some other discourses on chirality, see:
Potpourri
The Figure 8
Knot
Gentlemen's
Neckties
Molecular
Knots
Snails
and Crabs
Snails, Snakes and Darwin (html)
1
2
(pdf) 1
2
|

|
Read the stereoisomers
module in the StudyAids and do the
exercises. There is no need to record answers on your
homework.
Don't forget the Chirality
of Shells (Powerpoint). Do
left-handed whelks have a better survival rate than their mirror
image brethren? Click here.
|
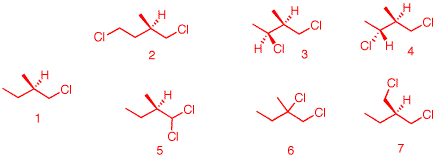
1. When
(R)-1-chloro-2-methylbutane undergoes free radical
chlorination, five dichloro constitutional isomers are
formed. What are these structures? Draw them. Be explicit as
to diastereomers, enantiomers, racemates, etc.
The four
constitutional isomers (same atom connectivity) are: 2, (3,
4), 5, 6 and 7. Still optically active: 6 (R), 3 (2S, 3S), 4
(2S, 3R) and 5 (R). Notice in 3 and 4, which are
diastereomers, the R, S-configuration has changed altough
the asymmetric center has not been altered. This is a change
in group priority. #6 is racemic because abstraction of the
tertiary hydrogen in the first propagation step creates a
planar radical. In the second propagation step, chlorination
occurs with equal facility on either face of the planar
radical. #7 is achiral; two identical groups,
-CH2Cl, in the molecule.
|
|
2. There are twelve possible Fischer
projections for a given enantiomer of ±-chloropropionic
acid. Six of the 24 of the total are shown below.
Assign R,S-configurations to each one. Draw the
remaining (S)-enantiomers.
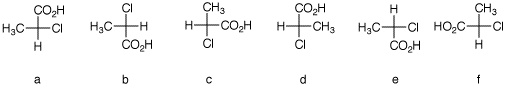
The configurations of
a-f are shown. Row 1 illustrates what the Fischer
projections mean. [See the end of the answer.]
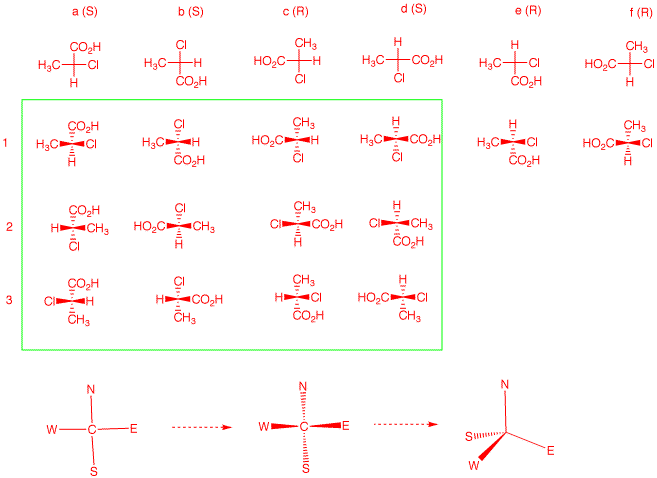
Consider the planar Fischer projection as a compass, NEWS.
Convert the compass to a Fischer projection. If you think of
N being in the rear, then W --> E --> S is clockwise.
Think of the Fischer projection as a seatless, three-legged
stool with WES as the legs and N as the spindle. Tip the
Fischer projection forward to do this. If you rotate about
the C-N axis, there are three different arrangements of the
legs. but SEW can all occupy the position of N; four
choices. For a given enantiomer of NEWS, there are 12
possible projections. Within the green box are the 12
projections for the case at hand. In 1 a-d, four different
groups are at N. Reading down the four columns a-d, one has
the three permutations 1-2-3.
|
3. A 3:1 mixture of enantiomers has
[α]D
= -60o. What is the rotation of the d- and l-enantiomers?
Show work.
The major enantiomer is
levorotatory based on the net negative rotation. The mixture is 3/4
levo (nb) and 1/4 dextro (na).
Thus,
obs. rotation =
na (x) + nb (-x) or, [na and
nb are the mole fractions; na + nb =
1]
-60 = 1/4 (x) + 3/4
(-x)
-60
= -x/2
x
= [α]D = -120 for the levo enantiomer; [α]D
= +120 for the dextro enantiomer.
|
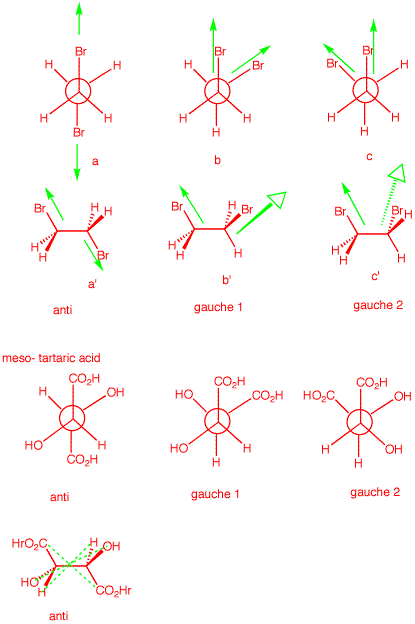
4. a) 1,2-Dibromoethane is optically
inactive yet it has a dipole moment. Explain and illustrate.
[Hint: Draw the staggered conformations and assess
optical activity and dipole moment for each.]
b) meso-Tartaric acid exists in three staggered
conformations, none of which has a plane
of symmetry. Yet the compound is
optically-inactive. Indeed, the only conformation that has a
plane of symmetry is quite unstable. Explain and
illustrate.
|
|
Optical activity is an
algebraically additive property; dipole moments are
cumulative in nature. The three staggered conformations of
1,2-dibromobutane are shown on the right in both Newman
projections and sawhorse views. The anti conformation has no
net dipole. The bond dipoles cancel. [Only the
green
C-Br bond dipoles are shown.] The two gauche
conformations have net dipoles. The vector sum of the bond
dipoles gives the molecular dipole. As to optical activity,
the anti conformation is achiral [center of
symmetry]. The gauche conformations are chiral and form
a racemate. No net optical activity.
|
|
|
5. Which of the following compounds are,
in principle, capable of resolution? Explain and illustrate.
[For 3-D Jmol views of these structures click here.:
5a,
5b,
5c,
5d,
5e,
5f.]
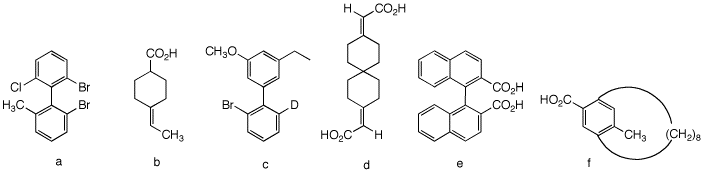
5a) This biphenyl is
not planar owing to the four large groups that inhibit
planarity. The two rings are orthogonal to one another
thereby producing two non-superimposable mirror
images.
b) This compound can
be resolved. Imagine that the CO2H group is above
the plane of the molecule. Draw its mirror image. They are
not superimposable. The doublebond and the 6-membered ring
are an extension of the cumulated double bonds in the
resolvable allenes.
c) Not resolvable.
Free rotation about the biphenyl bond is too rapid for
resolution.
d) This is an
extension of 5b. It is resolvable. An even nummber of
contiguous double bonds or 6-membered rings the compound is
resolvable; odd number, not resolvable.
e) Resolvable. Same as
5a.
f) Eight
CH2 groups in a row are just enough to span the
aromatic ring. Neither the CO2H nor the methyl
group can pass through the large ring. The compound is
resolvable.
|
|
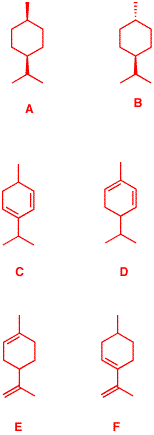
6. (S)-α-Phellandrene
([α]D
= +86o) is a monoterpene with the characteristic
fragrance of dill. (S)-±-Phellandrene
reacts with 2 moles of hydrogen gas in the presence of Pd to
give two cyclohexanes A and B, both of which
have the formula C10H20 and both of
which are optically inactive. Compound A has an
energy difference of 0.3 kcal/mol between its two chair
conformations while compound B has a value of 3.9
kcal/mol for the same equilibrium.
Explain the loss of optical activity, the energy
differences, and identify the structures A and
B.
|
|
6) You can look up the
structure of (S)-α-phellandrene. That two equivalents
of hydrogen are absorbed means that there are two double
bonds. [One triple bond is a possibility but there is no
where to place it.] At least one of the double bonds
must be in the ring and trisubstituted including either the
methyl or isopropyl group. This is true because two
cyclohexanes are formed on reduction. The group not involved
in the trisubstituted double bond is the center of
asymmetry. You can assume that the methyl and the isopropyl
group are 1,4 to one another because A and B are both
achiral [Need a plane of symmetry.] A must be
1,4-cis because it has the smaller energy difference. What
are two numbers whose difference is 0.3 and whose sum is
3.9.
x + y =
3.9
x - y =
0.3
2x =
4.2
x = 2.1 (isopropyl); y
= 1.8 (methyl)
Given the information
in the problem, there are only 4 possible structures for α
-phellandrene: C, D, E and F.
|
|
|
7.
(R)-α-Phellandrene
has been reported to have a specific optical rotation of
-217o. This observation suggests that the sample
of the enantiomer used in problem 6 above is contaminated.
Assume that the contaminant is the (R)-enantiomer and
that the (R)-enantiomer is pure. What percentage of
each enantiomer is present in the sample of problem 6? Show
work.
In #3 we solved
for the rotation. Here we calculate the % of each
enantiomer. Pure (R)-(-) = [α]D =
-217o; pure (S)-(+) = [α]D
= +217o; impure (S) = [α]D
= +86o.
obs. rotation = n+ (rot +) + n- (rot
-). Since n+ + n- = 1, then
n- = 1 - n+, or
obs. rotation =
n+ (rot+) + (1-n+)
(rot-), or n+ (rot+) -
(n+-1) (rot+), then factoring
obs. rotation =
(rot+)(2n+- 1), or
obs. rotation/(rot+) =
(2n+- 1) = ee (enantiomeric excess) = op (optical
purity)
op = ee = 86/217 = 0.396
= 40%
Solving for
n+:
n+ = (1 +
0.40)/2 = 0.70, then
n- = 1-
0.70 = 0.30.
(S)-α-Phellandrene
= 70%; (R)-α-Phellandrene = 30%
|






