Problem Set 2
Chapter 3, Alkanes
Due: Monday, September 21, 2009
 |
|
(This photograph is in the hallway across from 110 SCL)
|
Reading and Enrichment Assignments:
a. Work through How to Draw Cyclohexanes (PowerPoint)
b. The Conformation Module in the Study Aids will give you a good overview of the subject of conformation.
c. View The Evolution of Formulas and Structure in Organic Chemistry During the 19th Century (PowerPoint).
1. Redraw (line angle formula) and name (IUPAC) the hydrocarbon in this problem. For a dynamic view click here. For a static view click here. How to manipulate JSmol structures. [What if there are two different longest chains? Check here.]
2. Compound A (MW=139.19), a 1,4-disubstituted cyclohexane,
has the following composition: C, 69.03%; H, 9.41%; N, 10.07%. The
difference in conformational energy for the two chair conformations
of A is 0.4 kcal/mol. Using the A-value
data (Energy Differences Between ..... Cyclohexanes), determine the
structure of A. Illustrate and explain. What is the
conformational energy difference for the stereoisomer of A,
---namely A'. Explain and illustrate. Show the chair
comformations of A and A' with the appropriate
equilibrium arrows to illustrate the major and minor conformations.
Label each conformation with its energy.
3. Predict the heat of formation of n-nonane using the data presented
here.
Explain.
4. Using the heats
of formation tables, explain the difference
in the heats of formation of cis- and trans-1,3-dimethylcyclohexane.
The difference in the heat of formation of these stereoisomers is the
same as the difference in their heats of combustion. Justify
and illustrate with a diagram.
5. Calculate the heat of combustion of cyclopentane using the
data (ΔHfo of cyclopentane,
CO2 and H2O) in the heats
of formation tables. Compare your
value with the value in Table 3-5.
6. Draw Newman projections for the eclipsed and staggered
conformations of 2,3-dimethylbutane viewed along the
C2-C3 axis. Calculate the energy of each
conformation, both staggered and eclipsed.
|
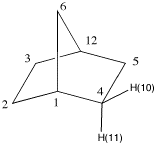
7. The structures on the right represent norborane. a) Provide its IUPAC name. b) Identify the bridgehead carbons by number. (The numbers on the far right are unrelated to the IUPAC numbering system.) c) Click on the Jmol logo, select "style", "labels" and then "with atom numbers". Only the numbered hydrogens appear. d) Measure the C(1)-C(6)-C(12) and the C(1)-C(2)-C(3) bond angles. (Click on "How to manipulate Jmol structures" for instructions on measuring.) e) Measure the corresponding H-C(2)-H and H-C(6)-H bond angles in d). What has happened to these bond angles as a consequence of the C-C-C bond angles? f) Measure the same angles for C(2) of the unstrained hydrocarbon, propane. g) Make a chart of the six measurements. Explain the trend. |
How to manipulate JSmol structures.
|
 |