1. In the E2 elimination of
2-bromo-2-methylbutane with NaOMe/HOMe, the ratio of
Zaitsev/Hofmann product is 70/30. when KO-t-Bu/HO-t-Bu is
employed, the ratio is 27/73. a) Calculate the selectivity for each
type of hydrogen in each experiment. b) Why is the the ratio different even
though the energy of the two products does not
change. c) How much heat is liberated in the
hydrogenation of each isomer? Construct a standard state
diagram to illustrate the thermochemistry. 2a) Using the Heat
of Formation tables, determine
the heat of hydrogenation of (Z)-2-butene. d) If cycloheptane and
(Z)-cycloheptene were strain free, what would be
there respective heats of formation? What would be the
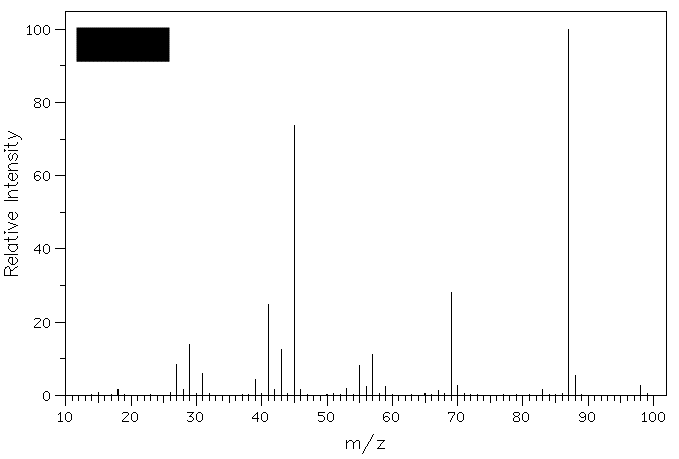
heat of hydrogenation? 3. Compound A (M+=88)
reacts with H2Cr2O7 to
form B. Compound B reacts with Grignard
reagent C to form D (M+=116).
Exposure of D to catalytic H2SO4
affords a single compound E (M+=98).
What are the structures A-E? Show your
reasoning.
b) Repeat part a) for (Z)-cyclohexene.
c) Knowing that cyclohexane is strain free (how do you
know this from its ΔHfo?),
what can be said about strain in (Z)-cyclohexene.
e) The ΔHfo for cycloheptane
is -28.2 kcal/mol and the ΔHfo
for (Z)-cycloheptene is -1.8 kcal/mol. What is the
heat of hydrogenation of (Z)-cycloheptene?
f) Draw a standard state diagram illustrating the three
hydrogenations b), d) and e). Your diagram should confirm
that the 7-membered ring compounds are less stable than
the strain free 7-membered ring compounds. Which
7-membered ring, (Z)-cycloheptene or cycloheptane,
is more responsible for the smaller heat of hydrogenation
relative to the 6-membered compounds. Illustrate and
explain.
g) (E)-Cycloheptene has never been isolated (put
in a bottle) but it has been detected as an intermediate
in reactions. Its ΔHfo has
been calculated as +18.7 kcal/mol. What is its heat of
isomerization to (Z)-cycloheptene? Show work.
h) What is its calculated heat of hydrogenation?
Compound A
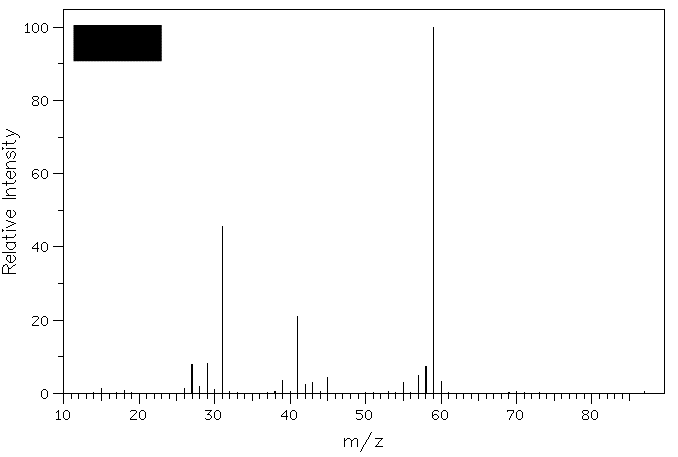
Compound D