Due: Monday, March 24,
2008
|
We have discussed Degree of
Unsaturation (D.U.) as it relates to hydrocarbons. How do
you find the D.U. if other atoms are present? Fear not!
There is an html
document and an independent,
different Powerpoint
presentation on this topic. The subject of oxidation
levels of organic compounds is a useful concept. Check it
out here Oxidation
Levels.The alcohol module in
ORGO
will give you a good review of some of the fundamental
reactions discussed in class and in Chapters 8 and 9.
1. How many grams of
K2Cr2O7 in aqueous
H2SO4 are required to oxidize 20
grams of cyclohexanol to cyclohexanone? [This is a
redox reaction from Gen. Chem. Derive the balanced
equation and show your work.]
|

Victor Grignard
(1871-1935)
Co-Nobel
Prize in Chemistry (1912)
|
|
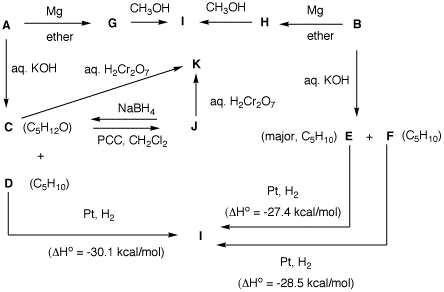
2. Two bottles on a shelf have had
their labels fall on the desktop. Both of the labels read
"C5H11Br". A student decides to run
some reactions on the contents of bottle A and
B to determine the structures of the two
compounds. She also has access, as do you, to
heats
of formation. From the flow
chart below, determine the structure of A and
B and identify C-I. [Note: The mixture
C and D is derived from A.] Show
your reasoning.
|
|
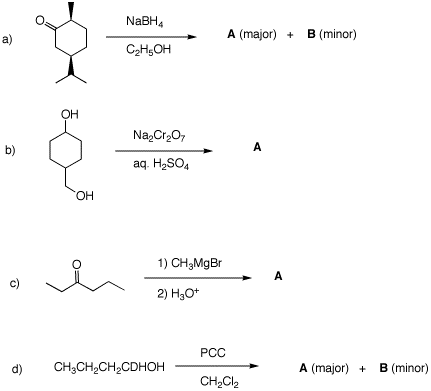
3. Predict the products in each of the
following examples. Justify your answer.
|
|
4. When organometallic reagents add to
aldehydes and ketones, there is only one addition.
Aldehydes give secondary alcohols and ketones afford
tertiary alcohols. Esters
(RCO2CH3), which are derivatives of
carboxylic acids and are at the same oxidation level,
undergo addition of an organometallic reagent (R'M) twice
to yield a tertiary alcohol, RR'2COH. The
reaction cannot be stopped after the first addition. It
is also true that carboxylic acids are at a higher
oxidation level than aldehydes by two electrons. Provide
an explanation for these results and provide a mechanism
for the process.
|

