Comprehensive Organic Chemistry -
Chem 225b
Problem Set 4
Chapter 5
Due: Monday, February 18,
2008

|
The
Borremean Rings
Versions of this symbol date to the time
of the Vikings.
In the 15th century, it was apparently the symbol of a
tripartite alliance of the Milanese families Visconti,
Sforza and Borromeo via intermarriage. Break any (wedding?)
ring and the others separate, hence the alliance is broken.
The rings form a chiral
object (left) that are not
superimposable on their mirror
image. A set of Borremean rings
has been used as the logo for a certain refreshment that
extols purity, body, and flavor. Is the sense of chirality
of the two sets of Borremean rings the same or different?
For some other discourses on chirality, see:
Potpourri
The Figure 8
Knot
Gentlemen's
Neckties
|

|
Read the stereoisomers
module in the StudyAids and do the
exercises. There is no need to record answers on your homework.
Don't forget the Chirality
of Shells (Powerpoint).
|
1. When (R)-sec-butylbenzene,
a.k.a 2-phenylbutane, undergoes free radical chlorination,
four monochloro constitutional isomers are formed, the
phenyl ring remaining intact. What are these structures?
Draw them. Be explicit as to diastereomers, enantiomers,
racemates, etc.
|
|
2. There are twelve possible Fischer
projections for a given enantiomer of lactic acid. Why?
(S)-(+)-Lactic acid, the cause of cramping after vigorous
physical exercise, was isolated from human muscle by
Berzelius.
Which of the following Fischer projections represent
(S)-(+)-lactic acid?
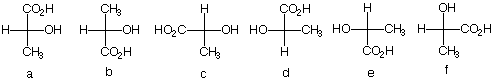
|
|
3. Enriched A has an optical
purity of 20% while B has an enantiomeric excess of
50%. Both compounds are rich in the (R)-enantiomer.
Compounds A and B react to form two
diastereomers
ARBR:ASBS
and
ARBS:ASBR.
One diastereomer is 64% optically pure while the other one
is only 33% optically pure.
a) How much of each enantiomer is present
in A and B? Show work.
b) What are the percentages of each
diastereomer? Show work.
c) Show how the ee of each diastereomer
is obtained.
d) What is the optical purity of each
diastereomer? Show work.
|
|
4. a) 1,2-Dibromoethane is optically
inactive yet it has a dipole moment . Explain and
illustrate.
b) meso-Tartaric acid exists in three staggered
conformations, none of which has a plane
of symmetry. Yet the compound is
optically-inactive. Explain and illustrate.
5. Which of the compounds on the right are, in principle,
capable of resolution? Explain and illustrate.
6. A mixture of enantiomers contains 1-1/2 times more of one
and shows [α]D -25.0o.
What are the rotations of the pure enantiomers? Which
one is in excess?
7. On the Lighter Side: The last eleven US Presidents
(33-43) are shown below in order of service in addition to
their party affiliation and their handedness.
[Handedness is "officially" designated as how you write.
Ford throwing out the "first pitch" indicates he is a
rightie and not a southpaw (leftie). By the way, no photo
has been converted to its mirror image by me but I found
Truman as both "enantiomers". ] Everyone of these photos
has something in common. Place that something (L or R) in
the last column and then separate the last two columns as
diastereomers and enantiomers. If you can correlate their
diastereoisomerism with party affiliation, you are better
than I am.
|

|
|
|
President
|
Affiliation
(D = Democrat; R = Republican)
|
Handedness
|
Chirality
|
|
|
|
|
|
|
Truman
|
D
|
L
|
|
|
Eisenhower
|
R
|
R
|
|
|
Kennedy
|
D
|
R
|
|
|
Johnson
|
D
|
R
|
|
|
Nixon
|
R
|
R
|
|
|
Ford
|
R
|
"R"
|
|
|
Carter
|
D
|
R
|
|
|
Reagan
|
R
|
R
|
|
|
Bush
41
|
R
|
L
|
|
|
Clinton
|
D
|
L
|
|
|
Bush
43
|
R
|
R
|
|
     
    
|












