Chem 221b
Problem Set 7
Chapters 20 & 21
Carboxylic Acids and Their Derivatives
Due: Monday, March 28,
2005
|
Esters
Esters are pleasant smelling, volatile
compounds. Isoamyl acetate (oil of banana), ethyl butyrate
(pineapple), and methyl salicylate (wintergreen) to name a
few. Apparently esters are pleasant to roaches.
The February 18 issue of Science (2005,
pg. 1104) reveals how entomologists and chemists unraveled the
structure of the sex pheromone of the ubiquitous German
cockroach, Blattella germanica.
|
Blattella
germanica
|
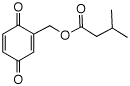
Blattellaquinone
|
|
1. Read the short paper cited above and
see what issues apply to what you have learned in organic
chemistry. The PDF version is better for general reading but
the HTML permits blow-ups of the nmr spectrum if you need to
measure coupling constants.
a) What was the source of the
pheromone?
b) How was blattellaquinone detected by gas
chromatography?
c) Account for the peak m/z = 180 in the EI mass
spectrum.
d) Given the 1H NMR data on the right, assign
each of the hydrogens (Ha-g) to a chemical shift
and explain the multiplicity and coupling patterns.
|
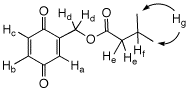
Jfg = Jef = 8.6 Hz
Jab = Jad = 2.0 Hz
Jbc = 10.0 Hz
|
Chemical Shift (δ)
|
|
0.786
|
|
1.920
|
|
1.989
|
|
4.721
|
|
5.916
|
|
5.943
|
|
6.356
|
|
|
2. Consider the synthesis of
blattellaquinone described in Fig. 2, pg. 1105.
a) Design a synthesis of isovaleroyl
chloride from isobutylene.
b) Design a synthesis of dimethylgentisyl alcohol
(2,5-dimethoxybenzyl alcohol) from benzene, methanol, and
paraformaldehyde as the only sources of carbon.
c) Why is the acid chloride preferrable to the anhydride of
isovaleric acid in the esterification?
d) DMAP used in the esterification is an acronym for
4-(N,N)-dimethylaminopyridine.
DMAP is a stronger base (~10-fold), and a better
nucleophile, than pyridine. Explain the enhanced reactivity
of DMAP. What is the ~pKb of DMAP?
e) The cerium reagent is a one electron oxidant. How many
equivalents of cerium reagent are required? What is the
oxidation level change for cerium (ceric ---->
cerous)?
f) During this oxidation two equivalents of methanol are
lost. Write a mechanism for this process.
|
|
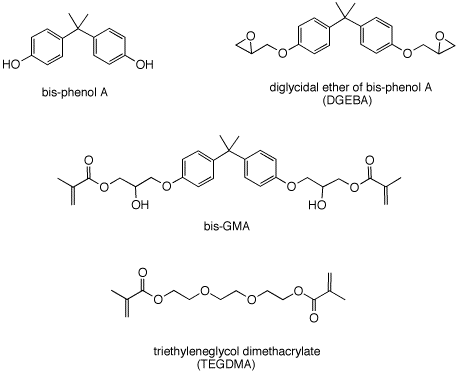
3. The polymerization of methyl
methacrylate forms Plexiglass. In the formation of modern
dental composites, the photo-initiator camphorquinone
(PS2)
causes cross-linked polymerzation of a mixture of bis-GMA
and TEGDMA.
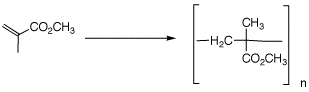
Polymerization of Methyl
Methacrylate
a) Show how bis-phenol A may be
prepared from phenol and acetone. Provide a
catalyst and mechanism.
Bis-phenol A is converted into DGEBA as shown on
page 630.
b) Is DGEBA a single compound? Explain.
c) What experiment could you design to demonstrate
that this is the mechanism and not direct
displacement on the carbon-chlorine bond?
d) Given methacrylic acid, how might you convert
DGEBA into bis-GMA.
|
|
|
|
4. You have access to compounds with
three or fewer carbon atoms, 18O water and all
reagents. Prepare the following compounds.
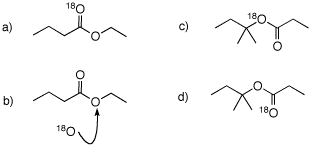
What are the products formed in the
aqueous acid hydrolysis of a-d? Account for the labeled
oxygen.
|
|
5. Complete each of the following
problems.
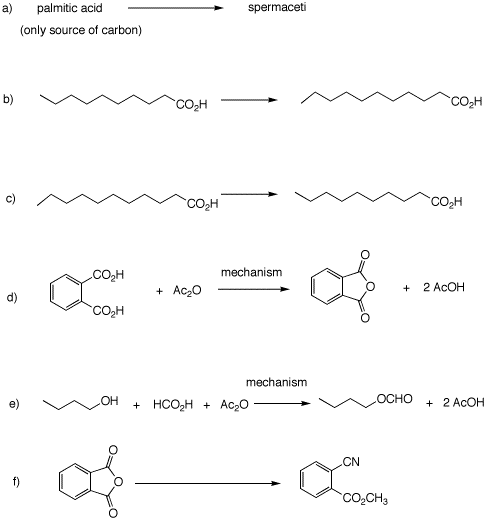
|






