Problem Set 6
Chapter 19
Amines
Due: Monday, March 21, 2005
Alkaloids [alkali-like] are
naturally-occurring bases. They can be toxic to animals
(Colombian dart
frog poisons (pumiliotoxins)),
beneficial to humans (quinine, malaria; ephedrine,
bronchodilators), and defensive agents for plants.
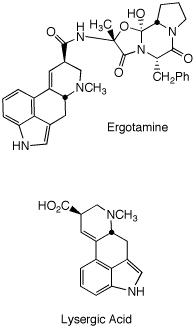
Ergotamine (left) is produced by the grain fungus
Claviceps
purpurea.
Ergot poisoning has been proposed as
the causative agent in the Salem witch trials. Ergot
alkaloids can be toxic to cattle and produce
hallucinogenic
effects in humans. Of course, synthetic derivatives
(LSD)
of lysergic acid have their own notoriety. Synthetic amine
pharmaceuticals include Valium
(diazepam, anxiolytic), Zoloft
(sertaline, antidepressant), and Prozac
(fluoxetine, antidepressant). There are some practice exercises in the
Amine and Nitrile modules of ORGO.
|
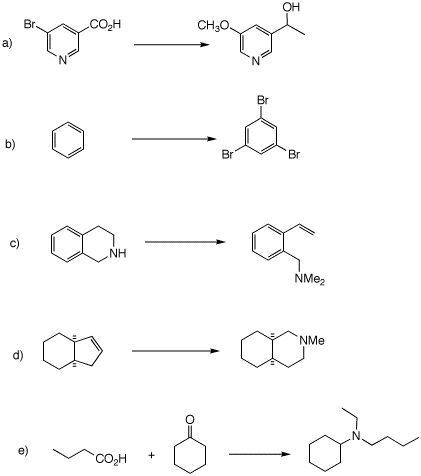
1. Provide efficient syntheses in each of the following problems. You may use additional sources of carbon in 1e. All reagents are available.
|
|
2. Do problem 19-52 on page 896. How does your answer compare with the one in the Solutions Manual. If there is agreement, then you are both partly correct. Explain what is missing from the answer. |
|
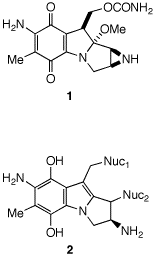
3. Mitomycin C (1) is employed in the treatment of solid cancers in hypoxic cells. [There is a discussion of quinones on pg. 838 and an incorrect structure of mitomycin C.] a) Of the four nitrogen atoms in mytomycin C, which one(s) is responsible for it being an alkaloid? Explain. b) Why are hypoxic cells suitable for the conversion of the quinone structure of 1 to a hydroquinone A? Write a mechanism for this process using electrons and protons. c) Hydroquinone A can lose methanol readily (1 can't) and form an indole B. Explain and write a mechanism. [Your answer should include a role for the nitrogen that becomes part of the indole ring.] d) Compound B is electrophilic at two "benzylic" sites toward nucleophiles. In this instance the nucleophiles are nitrogens in guanosine in doubly stranded DNA. Write a mechanism for the formation of 2 from B. e) Given that no stereochemistry is shown for the Nuc2 bond in 2, what can you infer about the mechanism in this step? |
 |
|
4. In the February 23, 2003 Sports Section of the New York Times an article by A. P. Grollman, M.D. appeared on dietary drugs and the effects of ephedra. In the case of Baltimore Oriole pitcher Steve Bechler, 23, the effect was lethal.. The active alkaloid in ephedra is ephedrine, (1R, 2S)-1-phenyl-2-aminomethyl-1-propanol. The picture on the right accompanied the article. (1S, 2S)-Pseudoephedrine, a diastereomer of ephedrine, is a common ingredient in decongestants. (The picture is used for educational purposes only. For a larger version click here.) a) What shortcomings appear in the Fischer projection and in the stylized representation of ephedrine? We have considered electron impact mass spectrometry (EIMS). Another technique is chemical ionization (CIMS), which usually displays molecular ions when EI may not. Compare the spectra of ephedrine via EI and CI shown here. b) What is the base peak in both spectra? Rationalize its formation. Why is m/z 107 not significant? c) Why is the molecular ion in the CI spectrum one mass unit higher? d) Is EIMS a useful technique for distinguishing between ephedrine and pseudoephedrine? Explain. |
|
|
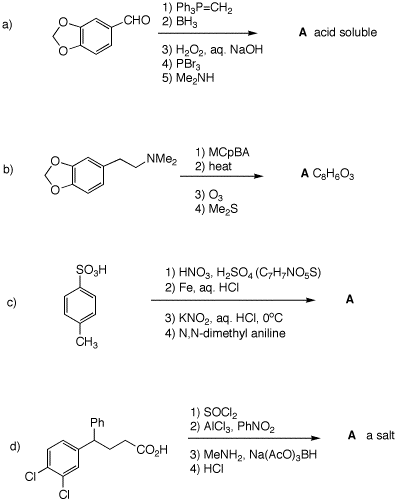
5. Provide structures for all compounds formed in the following reaction sequences.
|