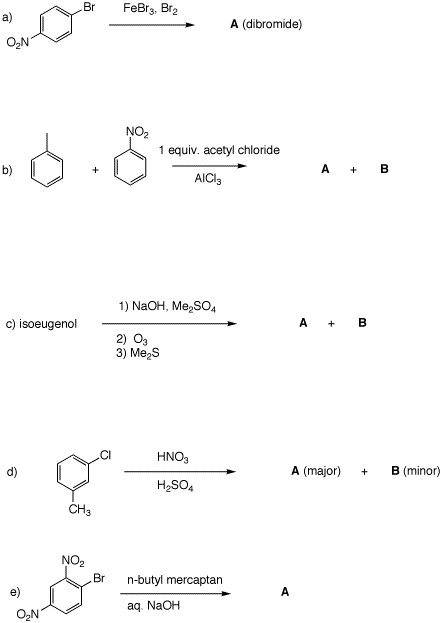
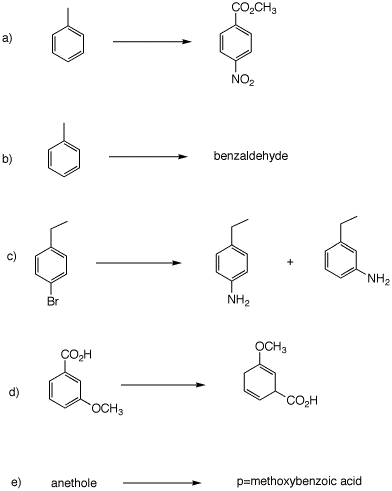
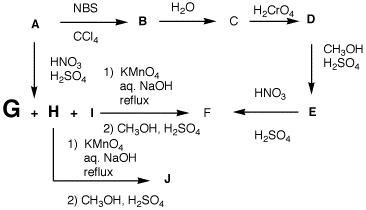
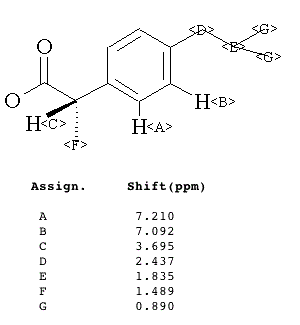Problem Set 4
Chapter 17
Due: Monday, February 14, 2005
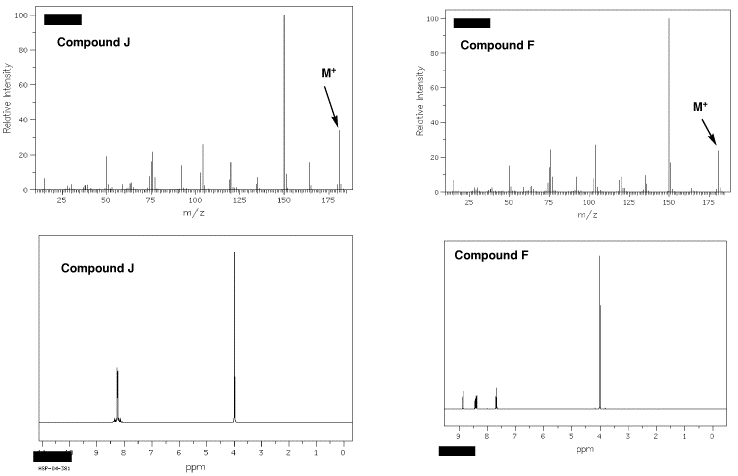
Nutmeg (Isoeugenol) |
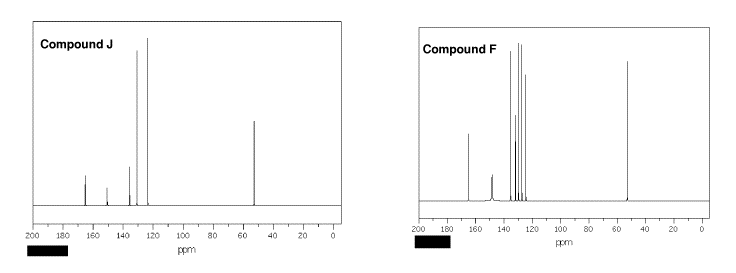
Clove (Eugenol) |
|
During the 16-17th century the demand in Europe for nutmeg (and its pod, mace) and clove as a flavoring, medicinal, amulet, and narcotic had a major impact on the course of world history. The sole source of these spices was the volcanic Spice Islands (the modern Moluccas in Indonesia). In a battle for domination of the area, the Dutch wrested the area from the Portuguese. To insure their exclusive hold on the spice trade, the Dutch bargained the new world settlement of New Amsterdam to the English in exchange (Treaty of Breda, 1667). The nutmeg is the seed that propagates the tree; seeds that could be spread to other islands by birds. To maintain control over and the price of the product, the Dutch chopped down new growth. Ultimately, clove and nutmeg were able to be grown in South America. The nickname for Connecticut, the Nutmeg State, is allegedly derived from the practice of Yankee peddlers foisting hand-carved, wooden nutmegs on unsuspecting clients. Nutmeg and clove can be toxic. Eugenol and isoeugenol are the principle aromatic compounds in clove and nutmeg, respectively. |