Chem 221b
Problem Set 1
Chapter 13
Due: January 24, 2005
221b Baker Street for help. Try the following practice problem. Click here. A solution is provided but ple-e-e-e-e-ase don't look at the solution until you are truly stumped.
1. The Novice Level in the NMR module is an interactive exercise that will help you learn about 1H and 13C NMR. The estimated order of complexity is 15, 11, 12, 2, 3, 7, 1, 10, 6, 8, 4, 5, 9, 13, 16, 14, and 17-20. Try to avoid the solutions until you are truly in need. These exercises are important. I hope to do some of them in class.
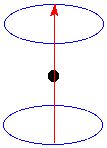

An α spin nucleus precessing in a magnetic field
How do I go about solving one of these "railroad" problems? I don't have a clue. You don't have to call (Chem)
2. Draw 1H NMR spectra for the three isomeric esters, ethyl acetate, methyl propionate, and n-propyl formate. Place the signals at their approximate chemical shift (the relative order should be correct), pay attention to multiplicity and area of the signals.
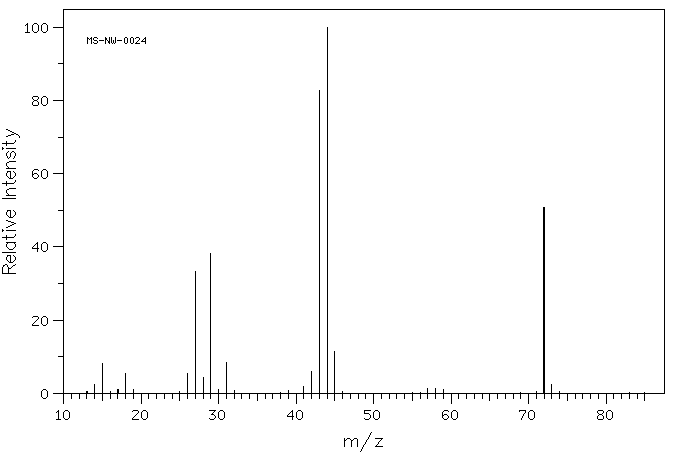
3. Compounds A, B, and C all have only an ethyl and vinyl pattern in their 1H NMR spectra. The 13C NMR and mass spectra are also provided. What are the structures of A, B, and C ? Show your reasoning?
Compound A:
Compound B:
Compound C:
4. Treatment of compound A with a Grignard reagent B produces compound C. Exposure of C to H2SO4 readily provides two compounds: D and (E)-E. Ozonolysis and dimethyl sulfide reduction of D affords F and G, while treatment of (E)-E with catalytic OsO4/HIO4 gives rise to H and I.
Compound A: 1H NMR: δ 1.17 (6H, d, J=6.94 Hz), 2.56 (1H, septet, J=6.94 Hz), 3.67 (3H, s). [see Fig. 1]
Compound F: See Fig. 2 and the blowups, Figs. 3 and 4.
Compound H: 1H NMR: δ 2.21 (3H, d, J=2.9 Hz), 9.79 (1H, q, J=2.9 Hz).
Compound I: 1H NMR: δ 1.04 (3H, t, J=7.3 Hz), 1.10 (6H, d, J=6.9 Hz), 2.48 (2H, quartet, J=7.3 Hz), 2.62 (1H, septet, J=6.9 Hz) [see also Figs. 5 and 6]
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Fig.5
Fig. 6
a) Assign the structures to A-I. Show your reasoning.
b) The spectum of compound F in Figs. 2-4 were recorded at 300 MHz. What is the value of the coupling constant J? Show work. Confirm your answer using both Figs. 3 and 4.
c) Explain the origin of the two most intense peaks in Fig. 5.
5. Determine the number of singlets present in the broadband decoupled 13C spectrum of each of the following dichlorides. Mark the identical carbons in each structure with a, b, c ...
6. In the 1997 Miramax film, Good Will Hunting, Minnie Driver's character Skylar did a considerable amount of grousing about organic chemistry in general and 1H NMR spectroscopy in particular. Her complaint about interpreting the proton spectrum of ibogamine has merit. There isn't a lot of information in it. Click here.
a) Look at the downfield region of the spectrum. What is the large singlet? What are the chemical shifts of the sp2 aromatic protons? How many of each are there?
b) What is the chemical shift, integral, and multiplicity of the highest field signal in the spectrum? Assign this signal.
c) What is the sign of the optical rotation of ibogamine shown here?
On the other hand, if her assignment had been to interpret the 13C NMR spectrum of ibogamine, she may not have been so bored. It is much more informative. Click here for the 13C NMR spectrum.d) What is the strong set of signals around 77 ppm? [It is actually 3 signals (triplet) and not 4 signals as the print out at the top of the spectrum indicates.]
e) Are all of the carbons of ibogamine present in the spectrum?
f) Draw the structure of laevorotatory ibogamine.
g) What enantiomer of ibogamine is Will Hunting (Matt Damon) drawing here?
h) Assign the singlets in the region 100-150 ppm to their respective carbons.That is, assign the taller signals (mark them with an "x" in part f.) to the appropriate group of carbons and the shorter signals (mark them with a "y") to their carbons. You will not be able to make a specific assignment of individual carbons.
i) Locate the carbons that absorb at higher field than 40 ppm. Mark them with a "z". How might they be described?
j) How many carbons remain unassigned? Describe the two types of carbons that remain. How many of each are there?