Chem 220a Problem
Set 8 Fall 2003
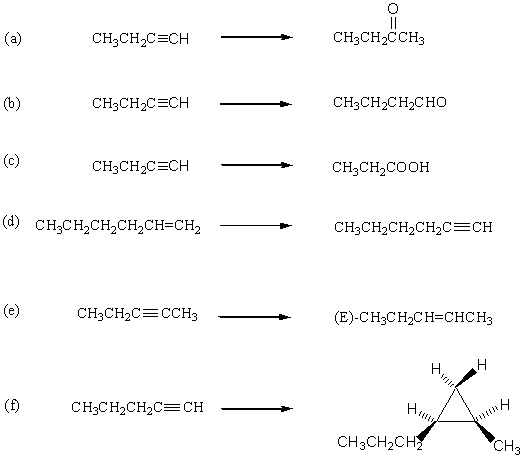
1. How would you carry out the following transformations? More than one step may be needed.
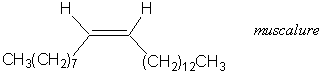
- Propose a synthesis of muscalure, the sex attractant of the common housefly, starting from acetylene and any alkyl halides.
- Hydrocarbon A has the formula C12H8. It absorbs 8 equivalents of hydrogen upon catalytic reduction using a palladium catalyst. Upon ozonolysis only two products are formed: oxalic acid (HOOC-COOH) and succinic acid (HOOCCH2CH2COOH). What is A? Explain concisely.
4. Compounds B and C have the formula C7H14. They are optically inactive; they are not resolvable, and they are diastereomers of each other. Catalytic hydrogenation of B or C yields D. D is optically inactive, but it could be resolved into separate enantiomers. Identify B, C, and D. Explain concisely.