Chem 220a Problem
Set 7 Fall 2003
1. Predict the products of the following reactions of 1-methylcyclohexene.

2. There are two dicarboxylic acids with the formula HO2CHC=CHCO2H. One dicarboxylic acid is called maleic acid; the other is called fumaric acid. In 1880, Kekulé found that on treatment with cold dilute KMnO4 maleic acid yields meso-tartaric acid and fumaric acid yields (±)-tartaric acid. Show how this transformation allows one to write stereochemical formulas for maleic acid and fumaric acid.
3. Suggest an efficient, multi-step preparation for each of the following compounds from the indicated starting materials. The compounds should be free of major amounts of isomeric side-products.

4. Provide a reasonable mechanism to account for the following observation.
![]()
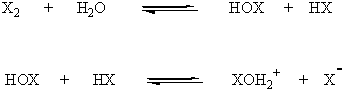
Note: When halogens are dissolved in water, hypohalous acids (HOX) are formed. Hypohalous acids are weak bases that can be protonated on oxygen to yield the strong electrophiles, XOH2+, which react with alkenes to yield halohydrins.