Chem 220a Problem
Set 1 Due
- Draw Lewis structures for the following molecules.
E.g.,
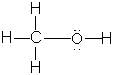
(a) methylamine, H3CNH2
(b) acetamide, H3CCONH2
(c) methyl azide, H3CNNN
2. Draw the principal resonance structures for:
(a) acetamide
(b) methyl azide
(c) pentadienyl cation, CH2=CH-CH=CH-CH2+
3. Identify the
hybridization for the non-hydrogen atoms in:
(a)
acetamide
(b)
capillin
(Wade, p. 370)
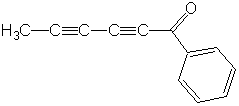
4. Order the following
compounds by increasing boiling point.
CH3CH2CH2CH2CH3 (CH3)4C
CH3CH2CH2CH2NH2 (CH3)2NCH2CH3
CH3CH2CH2CH2OH
5. Order the
following compounds by increasing dipole moment.
CF4 (CH3)3CH
(CH3)2CO (CF3)2CO
6.
Order
the following compounds by decreasing acidity (increasing pKa).
CH3COOH CF3COOH
CH3CH2NH3+ CH3CH2OH
CH3CH2NH2
7.
Identify
the functional groups that contain hetereoatoms in:
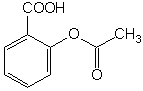
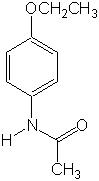
aspirin phenacetin