|

Raymond Davis Yale, '42, Ph.D (Chemistry). |
John B. Fenn Yale, '42, Ph.D. (Chemistry) |
1. Do the Alkyne Module in
ORGO. 3. Alkyne (R)-A does not
react with NaNH2 at the boiling point of
ammonia. (What is the boiling point of ammonia?) However,
stoichiometric NaNH2 at 150 oC
converts A to a new compound B, which, upon
the addition of water, liberates (R)-C.
'Hydrogenation' of C with D2/Pt gives
4-methylheptane (D) that is discenably optically
active. 4. Design an efficient synthesis of
4-octanone from compounds of three or fewer carbons. All
reagents are available to you. [Note: Efficient means
that you are not separating mixtures of compounds or
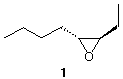
constitutional isomers.] 5. Design an efficient synthesis of
(±)-epoxide 1 from 2-butyne. All reagents are
available to you.
2. Two bottles are found on a laboratory shelf labeled
"alkyne A" and "alkyne B". Hydrogenation of
A or B over a platinum catalyst gives the
same alkane C. Compound A reacts with
H2 in the presence of
Lindlar's catalyst to form D. Compound D
reacts with O3 to form
a single compound E,
C3H6O.
On the other hand, compound B reacts with
Na/NH3 to give
F, which itself reacts with
Br2/H2O
to give a pair of constitutional
isomers, G and H. Treatment of either
G or H with aqueous NaOH gives the same
compound I, C6H12O, that is
also formed by the reaction of F with peracid.
What are the structures of A-I? Explain and
illustrate. [Note: G and H cannot be
distinguished Pay attention to
stereochemistry.]]
a) What are the structures A-D? Explain and
illustrate.
b) What does the optical active say about the reaction
A --> B?
c) Why is D optically active?
6. Provide the reaction conditions
A-S for the transformations shown below. provide a
brief commentary where stereochemical or related issues
are involved.