Chem 220a
Problem Set 4
Chapter 5
Due: Monday, October 7,
2001

|
The
Borremean Rings
Versions of this symbol date to the time
of the Vikings.
In the 15th century, it was apparently the symbol of a
tripartite alliance of the Milanese families Visconti,
Sforza and Borromeo via intermarriage. Break any (wedding?)
ring and the others separate, hence the alliance is broken.
The rings form a chiral
object (left) that are not
superimposable on their mirror
image. A set of Borremean rings
has been used as the logo for a certain refreshment that
extols purity, body, and flavor. Is the sense of chirality
of the two sets of Borremean rings the same or different?
For some other examples, click here.
|

|
|
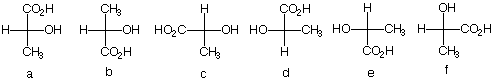
2. There are twelve possible Fischer
projections for a given enantiomer of lactic acid. Why?
(S)-(+)-Lactic acid, the cause of cramping after vigorous
physical exercise, was isolated from human muscle by
Berzelius.
Which of the following Fischer projections represent
(S)-(+)-lactic acid?
|
|
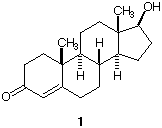
3. a) How many stereoisomers of
testosterone (1; [a]D
= +109o ) are possible? Explain.
b) What is the R/S designation for each
of the chiral centers?
c) If the hydroxyl group of 1 were
of the opposite configuration, would the compound still be
optically active? Would it be expected to have the same
melting point?
|
|
|
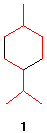
4. (S)-a-Phellandrene
([a]D
= +86o) is a monoterpene with the characteristic
fragrance of dill. Monoterpenes, which are C10
compounds, are dimers of two branched C5
compounds (structure 1).
(S)-a-Phellandrene
reacts with 2 moles of hydrogen gas in the presence of Pd to
give two cyclohexanes A and B, both of which
have the formula C10H20 and both of
which are optically inactive. Compound A has an
energy difference of 0.4 kcal/mol between its two chair
conformations while compound B has a value of 3.8
kcal/mol for the same equilibrium. Explain the loss of
optical activity, the energy differences, and identify the
structures A and B.
|
|
|
5. (R)-a-Phellandrene
has been reported to have a specific optical rotation of
-217o. This observation suggest that the sample
of the enantiomer used in problem 4 above is contaminated.
Assume that the contaminant is the (R)-enantiomer. What
percentage of each enantiomer is present in the sample of
problem 4? Show work.
|
|
6. A partially racemized compound (
[a]D=
48o) is analyzed by chromatography using a chiral
support and it is shown to be a 2:3
mixture. Why is this technique possible? What are the
rotations of the pure enantiomers?
Which one is the minor isomer? What is the
ee?
|
|
7. Free radical chlorination of
(R)-1-chloro-2-methylbutane leads to how many
constitutionally isomeric dichloro compounds? What are their
structures? Which ones, if any, form diastereomers? Which
ones are optically active? Which ones are optically
inactive?
|

