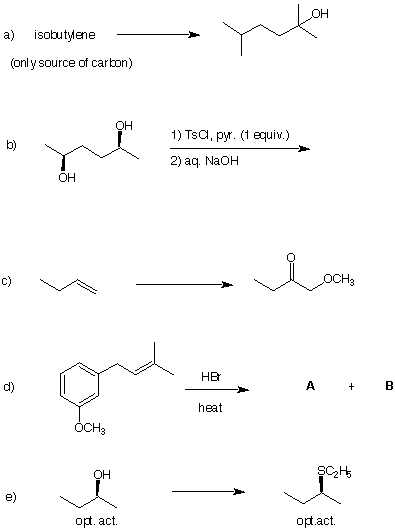
1. The two mass spectra shown below represent
constitutional isomers of C5H12. Which two
isomers fit these spectra? Explain. Predict the molecular ion and
the base peak in the isomer whose spectrum is not shown.
Spectrum 1a
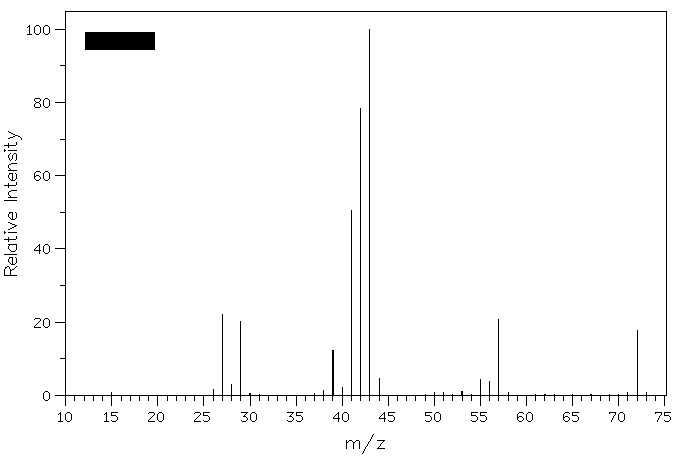
Spectrum 1b
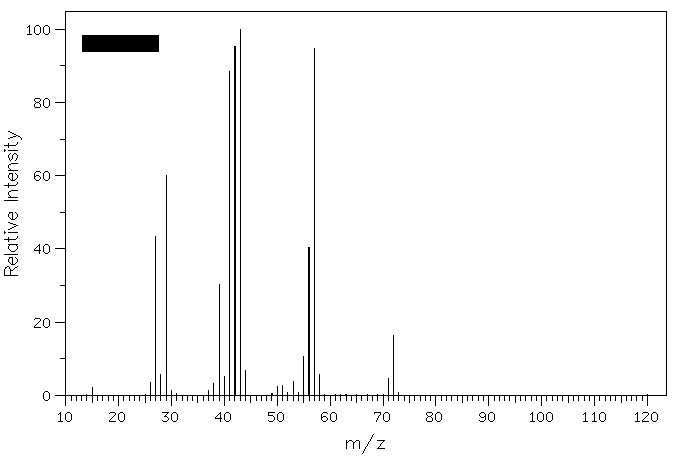
2. The mass spectrum shown on the right represents
methyl n-propyl ether, contrary to what is shown and explained on
page 619. What is the structure of the ion in the base peak shown
in the spectrum below. The spectrum on page 619 is that of diethyl
ether. The peak at m/z 59 represents what cation?
3. Lopressor (1), which is manufactured and sold as the racemate, is a b-adrenergic blocker. To prepare the (R)-enantiomer of Lopressor, (S)-epichlorohydrin 3 is treated successively with the phenoxide of 2 and then isobutylamine.
a) Would you prepare the phenoxide of 2 with aqueous NaHCO3 or NaOH? Explain. [pKa (H2O) = 15.7, pKa (phenol) = 10.2, pKa1(H2CO3) = 6.4]
b) Provide a mechanism for the formation of (R)-Lopressor from (S)-3.
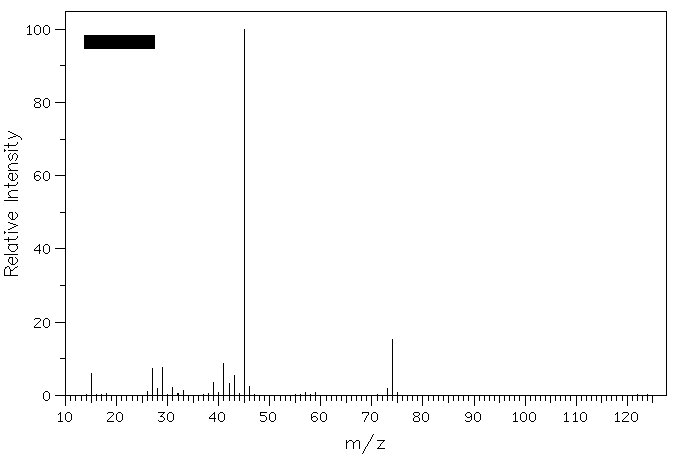
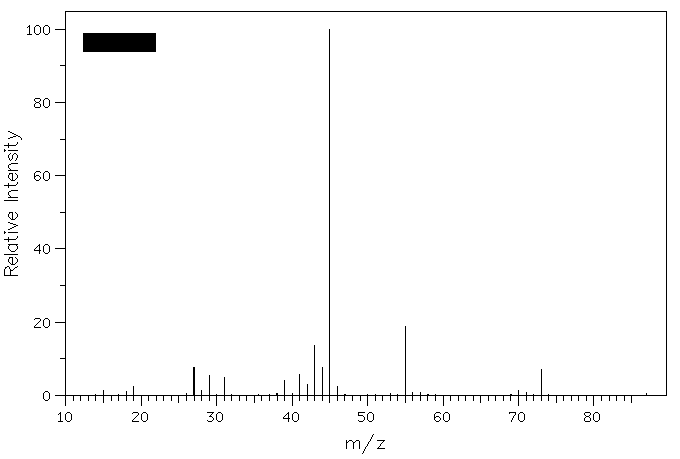
4. Epoxidation of (E)-2-pentene provides compound A. Reduction of A with LiAlH4 gives compounds B and C (C5H12O), whose mass spectra are shown below. What are the structures of A-C? Explain. Why is m/z = 73 less intense than m/z = 45 in the spectrum of compound C?
Compound B
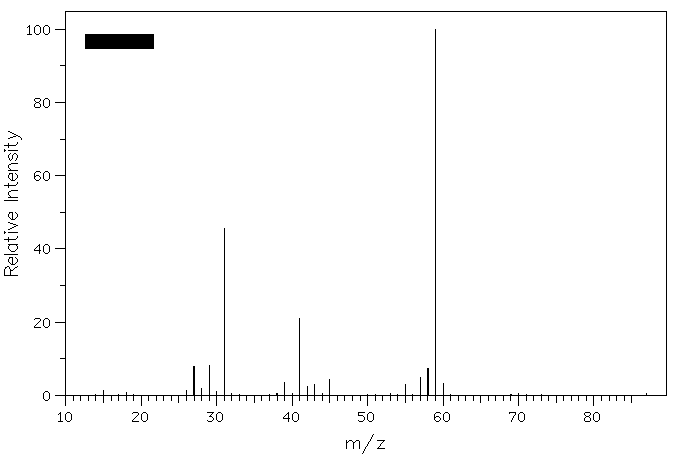
Compound C
5. 1,2,3-Trichloropropane is expected to have how
many molecular ions? What are their m/z value? What are their
relative intensities?
6. Provide the missing information in each of the following reactions.