Chem 220a - Organic Chemistry
Problem Set 1
Chapters 1 and 2
Due: Monday, September 16, 2002

John Dalton
(1766-1844)
|
John Dalton's formulation of an
Atomic
Theory in the first decade of the
19th century provided a theoretical basis for understanding
chemical behavior. In addition to defining the Law of
Multiple Proportions, he also formulated the Rule of
Greatest Simplicity, which held that water was a binary
compound, OH. (Note: Dalton did not use our modern symbols,
which came to us from Berzelius,
but rather
circles that were distinguishable
from one another.) Dalton established the combining masses
of H to O in water as ~1:6. This ratio was refined later to
1:8. The Rule
of Greatest Simplicity, which was
at odds with Gay-Lussac's
Law Combining Volumes of Gases, did not lead to a correct
formulation for the atomic composition of water. Moreover,
although there was agreement regarding the combining masses
of atoms in the first half of the nineteenth century, there
was
disagreement as to the unit mass
of the common atoms encountered in organic chemistry:
hydrogen (1), carbon
(2x6 or 1x12), oxygen (2x8 or
1x16). Since hydrogen was the lightest of the elements, it
was assigned a mass of one, a notion that is unrelated to
today's mass of hydrogen owing to the presence of a single
proton in the hydrogen nucleus. Berzelius's proposal of a
mass scale based upon O = 100 would have worked as well.
For a Brief History of Organic Chemistry
(PowerPoint), click
here.
|
1. Circle and name the functional groups (in
red) in
each of the following compounds. The inside front cover of your text
has a list of functional groups.
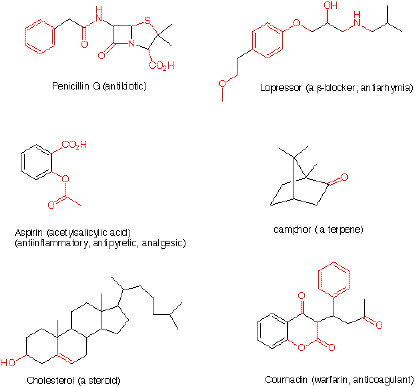
2. In the structure of cholesterol (problem #1),
how many primary sp3 carbons (methyl groups) are present?
How many secondary sp3 carbons (methylene groups)? How
many tertiary sp3 carbons (one hydrogen attached)? How
many quaternary sp3 carbons (no hydrogens attached)? What
is the molecular formula of cholesterol?
3. For each of the following acids or bases,
identify the corresponding conjugate base or acid.
a) NaNH2 (sodamide)
b) acetic acid
c) NaOH
d) CH3OH
e) CH3Li
4. Arrange the acids and conjugate acids #3 in
order of decreasing acidity (increasing pKa).
5. The palindromic year 2002 is not only the
sesquicentennial of Frankland's
studies on valence but also Williamson's
landmark work on the structure
of ether. Williamson prepared
unsymmetrical ethers such as
C5H11OC2H5 (n-amyl ethyl
ether; N.B.: amyl is a common name for a 5-carbon chain). Data from
Williamson's paper [Journal of the Chemical Society,
1852, 4, 229] is shown below. For the ether in
question, Williamson derived the empirical and molecular formula
C7H16O.
a)Using Williamson's calculated values, determine
the atomic masses of C, H, and O that he employed. Assume H =1.00
)
b) Using the masses in a), calculate the experimental percentages of
C, H, and O based upon the data presented: mass of sample,
CO2, and H2O. The amount of oxygen is
determined by difference.What experimental values do you get? Compare
your values with the published values.
c) Using the masses of a) and your percentages in
b), compute the empirical formula (assume it is the molecular
formula) of the ether. Is there a problem reaching the conclusion
that the ether contains 16 hydrogens?
d) Use the ideal gas law to show that d=PM/RT, where d = vapor
density and M = molecular weight. Use the experimental vapor density
[How many times heavier a given volume of vapor is compared with
air.] of the ether and the density of air (you should know the
composition of air from general chemistry) to show that the molecular
and empirical formulas are the same. All measurements are at the same
temperature and pressure.
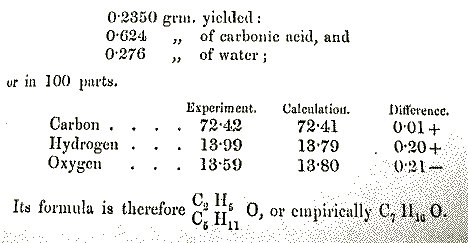
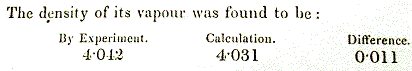
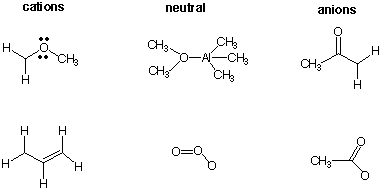
6. a) Draw Lewis structures for the species shown
below with all the appropriate electrons and charges.
b) For those structures that are
resonance stabilized, draw the two canonical resonance structures.
Use the formulas below, not Lewis structures.
c) For the anions, which one is the stronger
base? Explain
briefly.
7. Which of the following compounds have a net dipole
moment? Explain and illustrate.
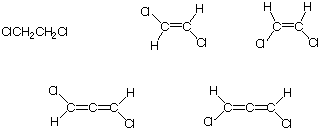
8. Draw a molecular orbital diagram for the last two structures in
#7. Are they identical or are they different. If they are different,
in what way are they different?

