|
1. The alcohol module in ORGO will give you a good review of some of the fundamental reactions discussed in class and in Chapters 10 and 11. The topic of oxidation levels of organic compounds is addressed in passing on pg. 458. We will consider the issue in more detail as described in Oxidation Levels. See also Web of Reactions-I. 2. The generic Grignard reagent, RMgX, is the conjugate base of a very weak acid, R-H (pKa ~45-50). Accordingly, Grignard reagents can deprotonate stronger acids than R-H. When methyl magnesium bromide reacts with 1-propyne, methane and CH3CCMgBr (1) are formed. However, 3-bromo-1-propyne (2) forms a normal Grignard reagent (HCCCH2MgBr) 3 with magnesium in ether without undergoing conversion to 1. Explain. [Hint: Consider the resonance structures of 3.] 3. Chemist Courtney needs to prepare a large quantity of n-propyl magnesium bromide in ether. She finds two cans of ether in the lab. Can A contains 600 mL and can B contains 100 mL of ether. She notices that can A is contaminated with 5% of ethanol from manufacturing while can B is free of ethanol. She is fully aware of the downside of having alcohols in the presence of Grignard reagents but she needs 500 mL of pure ether for her experiment. She has access to magnesium and n-propyl bromide but no more ether. How did she cleverly resolve her dilemma? |
Victor Grignard (1871-1935) Co-Nobel Prize (1912) |
|
4. You will remember that Alexander Williamson attempted to convert ethanol, via the agency of its sodium salt, into its higher homologue, n-butyl alcohol, by reaction with ethyl iodide. Instead, he formed diethyl ether. How would you, with the wisdom of 150 years and the aid of chapters 10, 11 and all that has preceded them, accomplish Williamson's objective? |
|
5. Compound A, C8H16, undergoes hydroboration and alkaline peroxide oxidation to provide B, C8H18 O. Treatment of B with excess Jones' reagent gives C, C8H16 O. Ozonolysis of A affords a single compound D, C4H8 O. D is converted into E, C4H8 O2, upon treatment with Jones' reagent. Compound F, C4H8 , yields two products, G and H (a ketone) upon ozonolysis. Hydroboration and alkaline peroxide oxidation of F gives I, which, upon reaction with PCC, affords D. What are the structures of B-I? Explain and illustrate. What structures satisfy A? How can you distinguish between your options for A? |
|
6. How many grams of CrO3 are required to oxidize 1.5 moles of cyclohexanol? Show work. |
|
7. Each of the following chemical transformations requires more than one step. Provide solutions.  |
|
8. 2-Undecanone 1 is alleged to be offensive to cats and supposedly stops them from doing their business where you don't want them doing it, like in your garden. Well, I tried it many years ago and it didn't seem to work, that or this neighborhood tom was not easily offended. In any case, design a synthesis of 1 from oleic acid 2 and acetaldehyde. A really good plan will use all of the carbon atoms of oleic acid. [Hint: 2 + 9 = 11; This problem is mainly an exercise in Grignard reagents.] 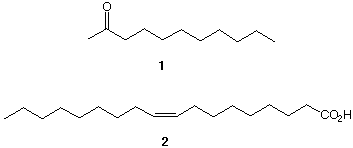 |
|
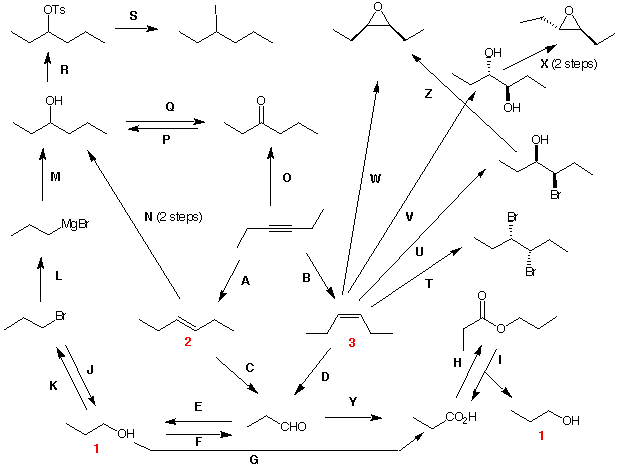
9. You have been accumulating many reactions during the term. A good way to practice with the reactions is to create a network starting from a particular compound, in this case 3-hexyne, and keep doing reactions with reagents creating compounds. In this exercise you are asked to provide the reagents A to Z, i.e., reaction conditions, to complete the network of structures. Each letter is one set of conditions unless noted otherwise. For structure 2, (E)-3-hexene, what are the stereoisomers provided by reagents T - Z? For more practice of this type using oxidation levels, try the Web of Reactions I, or the Web of Reactions II. |
|
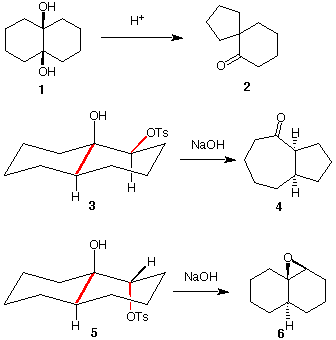
10. 1,2-Diols can be induced to undergo interesting molecular rearrangements of the pinacol type (pg. 479). The rearrangement of cis-diol 1 to spiroketone 2 is such an example. Provide a curved arrow mechanism for this reaction. The same reaction can be base-induced by first converting the cis-diol precursor of 3 to the monotosylate 3. [Note: The secondary alcohol forms the tosylate, not the more hindered tertiary alcohol]. Provide a mechanism for the conversion of 3 ---> 4. Your focus should be on the alignment of the red bonds. The stereoisomer of 3, namely, tosylate 5, provides the epoxide 6 and not the product of rearrangement. Again, focus on the red bonds.
 |
