|
1. The alkyne module in ORGO will give you a good review of some of the fundamental reactions of alkynes. 2. Of the following bases, which ones will deprotonate 1-butyne greater than 90%: C2H5ONa,, C6H5Li (PhLi, phenyllithium), CH3NHNa, NaCH2SO2CH3, and CH3Li. Explain the your reasoning. Consult the pKa table. 3. Compare the heats of formation of (Z)-2-butene and (Z)-2-pentene. Do the same for the (E)-isomers. From this information and given the heat of formation of 2-butyne, estimate the heat of formation of 2-pentyne. Compute the heat of hydrogenation of 2-pentyne ---> (Z)-2-pentene ---> n-pentane for each of the steps. Draw a diagram relating the standard states to each of the components. Which reduction is more exothermic, yne --> ene or ene --> ane? 3. When cyclododecyne (C12H20) is treated with NaND2 in ND3, cyclododecyne (C12D20) is isolated. Explain and illustrate. See pg. 395. |
Sterling Chemistry Laboratory |
|
4. Alkyne A (C8H14) reacts with H2 in the presence of catalyst B to give n-octane. [Although B may be several things, it is incapable of selective hydrogenation. Pick one.] Treatment of A in the presence of catalyst C produces D (C8H16). The reaction of compound D with reagent E produces meso compound F (C8H18O2). What are the reagents and structures of A-F? Explain and illustrate. How are the stereoisomers of products D and F, namely, D' and F', prepared from A? Explain and illustrate. |
|
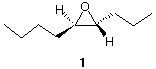
5. Devise a synthesis of (±)- epoxide 1. Your source of carbon atoms are 2-butyne, 1-propene, and acetylene. You must use each one once, and only once. All reagents are available to you. [Hint: It is best to work the problem backwards by first thinking abourt how you make 1 from its immediate precursor, etc., etc., until you make the connection. Both 1-propene and 2-butyne cannot be used as is.] Why is your product 1 racemic as opposed to being the 4R, 5R enantiomer shown? |
|
|
6. When 1,1-dichloropentane is successively heated at 200 oC with excess KOH, cooled, and poured into water, 2-pentyne is isolated. When the same experiment is conducted with NaNH2 at 150 oC, 1-pentyne is the product. Illustrate and explain. |
|
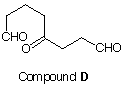
7. Two bottles, A and B, in a laboratory bear only the inscription "pure C10H16" on their labels. To determine the structure of the contents of each bottle, a chemist conducts the following experiments. She hydrogenates compound A over platinum and obtains C, which proves to be identical with an authentic sample of ethylcyclooctane. When A is subjected to ozonolysis followed by treatment with dimethyl sulfide, compound D is formed. Compound B does not react with NaNH2 in NH3 at the boiling point of ammonia (-33 oC) but it does undergo reduction with Na/NH3 to give E, C10H18. In addition, hydrogenation of B over Pt forms n-decane. When she ozonizes E, she isolates a C6 dialdehyde, F. From these experiments she is able to determine the structures of A and B although there is something about A that she cannot conclude from her research. Moreover, there was a compound G also formed along with C-F whose structure she inferred. What were her structures for A-G? Explain and illustrate. |
|
|
8. When 1-hexyne is heated with KOH at 200 oC, more 2-hexyne (DHfo = +25.7 kcal/mol) is expected than 3-hexyne (DHfo = +25.2 kcal/mol). Explain. |