|
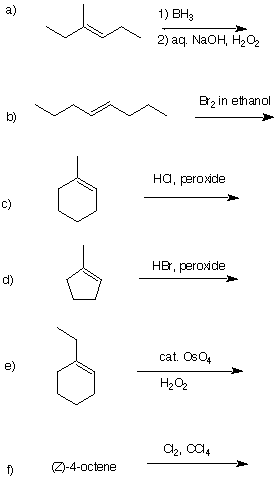
1. The alkene module in ORGO will give you a solid review of Chapter 7 and also introduce you to concepts covered in Chapter 8. After the reading do problem #6 first before tackling 2-5. You should also read the ozonolysis module. How do I approach solving problems like #2? Here is a step-by-step analysis of #2 PS7 from F2000. [Note: All ozonolysis reactions in 2-4 include a reductive treatment with dimethyl sulfide.] 2. An optically active compound A, C8H17Br, reacts with sodium ethoxide in ethanol to afford Saytzeff products B (C8H16) and (S)-C (C8H16). Compound B reacts with OsO4 to give a meso compound D while ozonolysis of B gives a single four carbon ketone E. Ozonolysis of C provides a ketone F and aldehyde G. What are the structures a A-G? Explain your reasoning. Assign R/S configurations to all centers of chirality. [Hint: Solve for B before C. C has a stereoisomer (S)-C' . Why is none, or very little of (S)-C', formed?] |
Vladimir Vasilovich Markovnikov (1838-1904) |
|
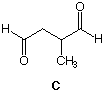
3. Optically active compound A, C10H16, reacts with H2 in the presence of Pt to form optically inactive B, C10H20. The heat of hydrogenation is 57.2 kcal/mol. [Hint: pg. 307] Ozonolysis of A produces (R)-dialdehyde C. What are the structures of A-C? Explain. |
 |
|
4. Compound (S)-A, C6H12, liberates ~30 kcal/mol of heat upon hydrogenation to give optically inactive B. Treatment of A with HBr forms three bromides, namely, optically inactive C and optically active D and D', both having the formula C6H13Br. Compound D is of the (S, S)-configuration. Compound C reacts readily with water to form E, C6H14O. E2 elimination of C forms two compounds (E) and (Z)-H and I but no A. Compounds H form aldehyde J and ketone K on ozonolysis while I forms a C5 ketone L and M. When A reacts with HBr in the presence of a peroxide, optically active bromide F, C6H13Br, is formed. Treatment of F with aqueous NaOH gives mainly optically active G, C6H14O, and a small amount of A. Hydroboration of A followed by oxidation with alkaline peroxide also gives G. What are the structures of A - M. Explain. Provide a mechanism that explains why more of (E)-H is formed than (Z)-H. [Hint: The structure of A is in the first sentence!] |