Problem Set 5
Chapter 6
Due: Monday, October 15, 2001
|
1. Study #2 and #3 in the Alkyl Halide module and #1 in the Ether module in ORGO. 2. Consider each of the compounds 1 and 2
as a racemate. 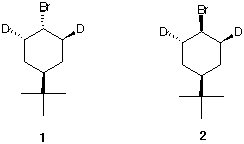 a) What is their relationship to one another? |
R. S. Cahn (left), C. Ingold, and V. Prelog (1966) [One wag has suggested that "R" and "S" were chosen by Cahn for the CIP system for an obvious reason.] |
What is the common configuration (R,S) at these carbons? The carbons bearing the Br- and t-butyl groups are not chiral (why?) but they are stereogenic. Explain. [Hint: Try to use R and S on the carbons bearing the Br- and t-butyl groups. Any luck? Try inverting the configuration at the carbons bearing the Br- and t-butyl groups. What happens?] |
|
3. Predict and explain the formation of each of the products of the following reactions. Be sure to address issues of stereochemistry and optical activity. |
|
4. An optically active compound (R)-A, C5H11Cl, undergoes free radical chlorination to give 5 constitutional (structural) isomers (C5H10Cl2), one of which is a pair of diastereomers. Dichlorides B and C are optically inactive while the remaining four, D, E, F, and H are optically active. The pair of diastereomers is D and E. The carbon bearing the new chlorine atom in E is of the (S)-configuration. Compound C reacts more rapidly with water than any of the dichlorides and forms compound G, C5H11ClO. Dichloride H is geminally substituted. What are the structures of A-H? Provide R/S configurations for D, E, F, and H. Include explanations for the following: a) Why are B and C optically inactive?, b) Why does C react rapidly with water?, c) Is G optically active? Explain. |
|
5. Rotation about the double bond of an alkene does not
occur readily. Thus, 1 and 2 are geometrical
stereoisomers that have different properties. When (3S,
4R)-4-bromo-3-methylheptane (A) reacts with
C2H5ONa in ethanol, a trisubstituted
alkene (olefin) B is formed as well as a trans
disubstituted alkene C. 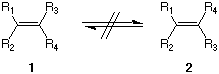 a) What are the structures of B and C and how are they formed? Show the mechanism. A diastereomer of A (A') reacts with C2H5ONa in ethanol to provide a stereoisomer of B (B') and the enantiomer of C (C'). c) What is a structure for A'? Assign R/S configurations. Does it matter which enantiomer of A' you use? Explain. |
|
6. Let us revisit PS3 #3. a) Which monochlorinated constitutional isomers are achiral? |
|
7. In section 6-19, pg. 268 of your text, the reaction of hydroxide with (±)-2-bromobutane to form 1-butene (19%) and 2-butene (81%) is illustrated. a) How much of each alkene is derived from each of the enantiomers of the alkyl bromide? The 2-butene is comprised of cis-2-butene and trans-2-butene (see pg. 273)? b) These geometrical stereoisomers do not interconvert. Why? The heat of formation of the cis isomer is -1.9 kcal/mol while the trans isomer is -3.0 kcal/mol. c) Which isomer is more stable? |