Material in this Problem Set will be covered in the final exam.
|
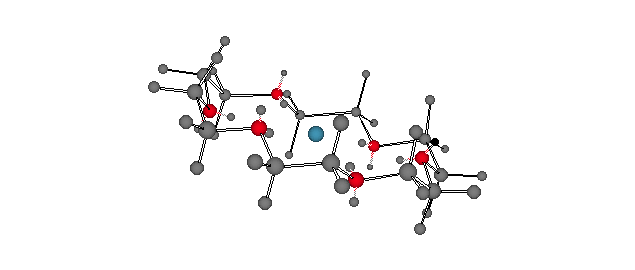
Potassium cation solvated by the cyclic polyether, 18-crown-6 [18-membered ring; 6 oxygen atoms]. Each of the ethano groups is in a staggered conformation with each of the O-C-C-O dihedral angles at ~60o [gauche]. For a ChimeTM version, click here. Note that the six oxygen atoms occupy the same spatial arrangement as do the six carbon atoms in chair cyclohexane. The discovery of the crown ethers by Charles Pedersen of DuPont earned him a share in the 1987 Nobel Prize in Chemistry. |
|
1. Read about the history of, and Williamson's contribution to, the structure of ether. There is also a Powerpoint version. |
|
2. The two mass spectra shown below represent two compounds with the formula C4H10O. Compound A (MS-NW-0071) can be prepared in only one way by the Williamson procedure while compound B (MS-NW-1331) is accessible by two routes using the Williamsom method. On the other hand, compound A is readily prepared as described by Valerius Cordus whereas the preparation of compound B by this method is not as efficient. There is a third possible compound that fits the formula C4H10O and the functional group under consideration. What are the structures of A, B, and, C? Justify your answer. How does your answer compare with the explanation provided in your text? |
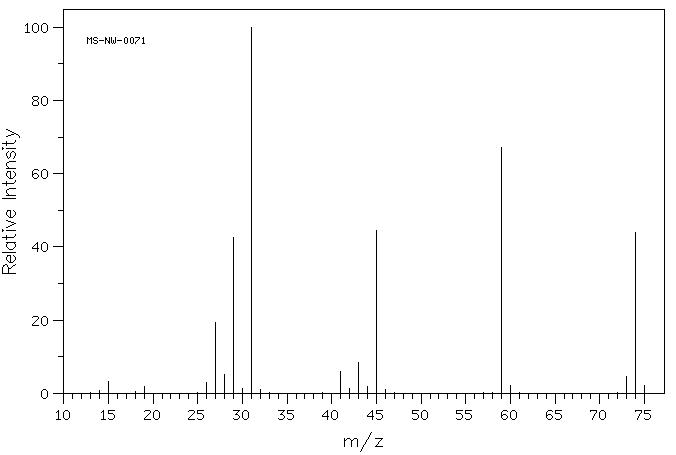
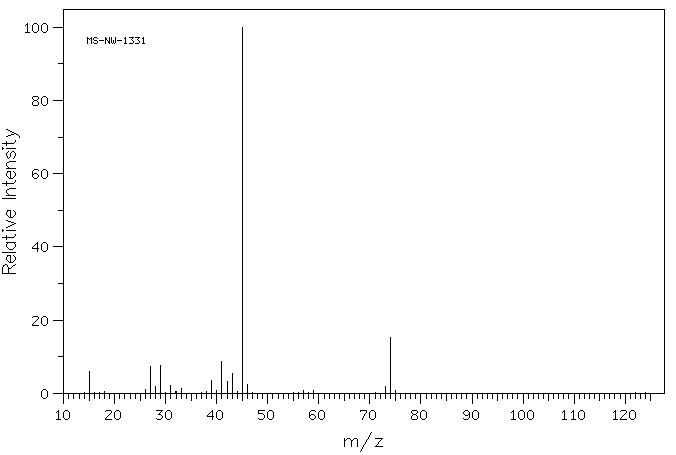
|
3. Diethyl ether has a propensity to form hydroperoxides and peroxides (pg. 625) when exposed to oxygen. The initiation step involves breaking a C-H bond with O2. [Note: The double bond of O2 does not have paired electrons.] a) Write a radical chain reaction to form the hydroperoxide of diethyl ether. b) Use your knowledge of radicals to compare the relative reactivity of t-butyl methyl ether, diisopropyl ether, and diethyl ether toward the initiation step. Rank the 3 ethers in order of relative reactivity. Justify your answer. c) When the hydroperoxide of diethyl ether is treated with dimethyl sulfide, dimethyl sulfoxide, ethanol and acetaldehyde are formed. Provide a mechanism for this reaction. [Hint: Think about ozonolysis.] |
|
4. Squalene (pg. 633) is converted enzymatically into chiral squalene-2,3-epoxide. The epoxide undergoes cationic cyclization to the intermediate shown below which, in turn, rearranges to lanosterol. Lanosterol itself is converted biologically into cholesterol. a) What is the R, S-configuration of squalene-2,3-epoxide? b) Write a mechanism for the cationic cyclization of squalene epoxide. You can initate the cyclization with H+. You need not worry about stereochemistry but do attach the correct carbon atoms together. You will notice that Markovnikov's Rule is not obeyed in the formation of one of the carbon - carbon bonds. Which one is it? c) One of the 6-membered rings of the intermediate is fixed in a boat conformation. Which one is it? Why? d) Show how (curved arrow formalism) the intermediate's red atoms and electrons are rearranged into the red atoms of lanosterol with loss of a proton. [Note: Pay attention to which red atoms are above and below the plane of the rings, i.e., the black atoms.] |
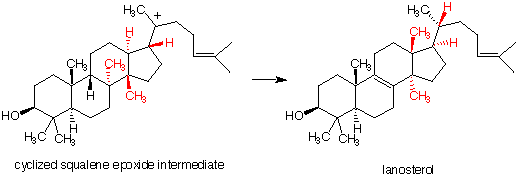 |
|
5. The formation of the epoxyresin prepolymer from bisphenol A and epichlorohydrin employs racemic epichlorohydrin (pg. 638). If (R)-epichlorohydrin were used in the formation of the prepolymer, the prepolymer would be of the (S,S)-configuration at the stereogenic epoxide carbons. Explain. |