Chem 220a - Organic Chemistry
Problem Set 1
Chapters 1 and 2
Due: Monday, September 17, 2001

|
John
Dalton (1766-1844)
Made important contributions to early atomic theory
[Law of Multiple Proportions]. Unfortunately, his
'rule
of greatest simplicity' led to the idea that water was a
binary compound (two atoms) caused confusion in the ensuing
years.
|

|
G.
N. Lewis (1875-1946)
Developed the concept of the covalent bond and the octet
rule. An early version of the octet rule had the electrons
located at the corners of a cube.
|
1. Draw Lewis structures for methyl
acetylene (CH3CCH) and allene
(1,2-propadiene,
CH2CCH2), the tautomeric components of
MAPP gas. Illustrate and explain the
tautomeric relationship.
2. Illustrate resonance in diazomethane
(CH2N2; this formula not only indicates
composition but also atom connectivity) and in acetate ion.
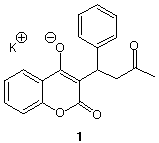
3. Draw two other resonance structures of the anion of the
anti-coagulant drug, coumadin 1 (warfarin). Draw the three
possible tautomeric forms of the conjugate acid of coumadin. Can the
anion of coumadin be converted to its conjugate acid with acetic
acid? [To assist you in this part of the question, assume that
the conjugate acid of 1 is protonated on carbon. Name and
circle the two proximate functional groups. Use the pKa
table to find an appropriate model for the conjugate
acid.]
[Historical Note:
The development of coumadin (warfarin) as an
anticoagulant follows from the isolation of dicoumarol
from rotting sweet clover after a Wisconsin farmer noted
that his cattle were bleeding
and dying. The research that led to the development of
warfarin was sponsored by the
Wisconsin
Alumni
Research
Foundation (WARF), hence its
name. (see
WARF History at this site)].
4.
|
In a historical paper in 1852, Williamson reported the
preparation of an unsymmetrical
ether. This new ether had a vapor density approximately
twice that of air. A combustion analysis was conducted.
"... the ether being burnt by oxide of copper, the
following results were obtained:
0.2215 grm. of liquid gave:
0.482 " " carbonic acid [CO2], and
0.2685 " " water ..."
From these data, determine the molecular formula of this
ether and draw its structure. Show your work. [Remember:
Oxygen is not determined directly in a combustion analysis
but rather by difference. The ideal gas law relates
molecular weight and gas density at the same temperature and
pressure.]
|

Alexander
Williamson
(1824-1904)
|
5. Isoprene (2-methyl-1,3-butadiene, 1) is an important
monomer in the formation of essential oils (terpenes: camphor,
b-carotene, etc.) and rubber. Draw a
molecular orbital representation of isoprene in the conformation
shown below. Label the hybridization (sp, sp2,
sp3) of all carbons and label s-
and p-bonds.
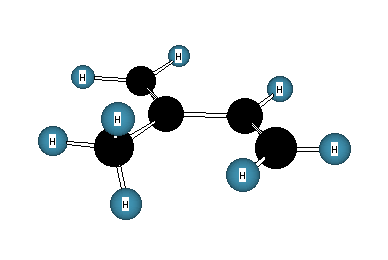
1
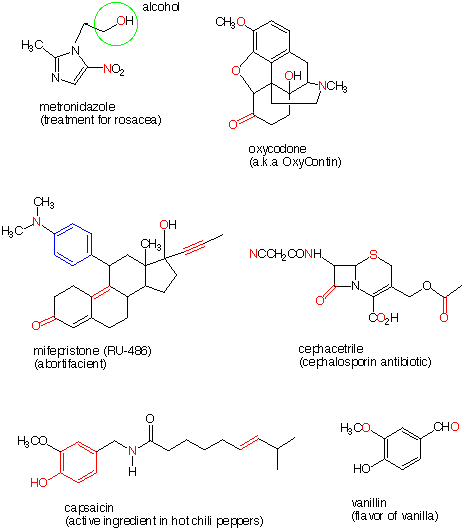
6. In each of the following structures certain atoms, or groups of
atoms, are highlighted in red or
blue. Either print out the structures,
or better yet, draw them, and circle each of the functional groups in
which each atom, or group of atoms, is contained. Write the name of
the functional group next to each circle. For example, the
red oxygen in metronidazole is part of
an alcohol, -CH2OH. Note that
the lefthand side of the structures of capsaicin and vanillin are the
same, but what a difference your taste buds discern!
SKYLAR: Well, have you studied organic chemistry?
WILL: A little bit.
SKYLAR: Oh, just for fun.
WILL: Yeah, for kicks.
SKYLAR: Yeah, it's SO much fun studying organic chemistry. Are you
mad? Have you completely lost your
mind? Nobody studies it for fun. It's not a necessity, especially
for someone like you. .....Good Will Hunting, Miramax Films





