|
1. The alcohol module in ORGO will give you a good review of some of the fundamental reactions discussed in class and in Chapters 10 and 11. 2. Ethyl bromide reacts with Mg in ether to form the Grignard reagent, ethyl magnesium bromide. However, 1,2-dibromoethane reacts with Mg in ether to form ethylene. Explain and illustrate. 3. In an effort to prepare 1,5-hexanediol A, a novice chemist reacts 4-bromo-1-butanol B with Mg in ether. Subsequently, an aldehyde C is added to the reaction mixture. After water is added to the reaction mixture, no diol A is isolated, but aldehyde C is recovered in addition to the isolation of D (C4H10O). What are the structures of A-D? Explain and illustrate. 4. Compound A (C6H12O2) reacts with two equivalents of C2H5MgBr to form B (C7H16O) and C (C3H8O). B reacts readily with H2SO4 to form only one possible compound D (C7H14). Ozonolysis of D affords E (C2H4O) and F (C5H10O). Na2Cr2O7 in aqueous H2SO4 reacts with some of these compounds in the following |
Victor Grignard (1871-1935) Co-Nobel Prize (1912) |
|
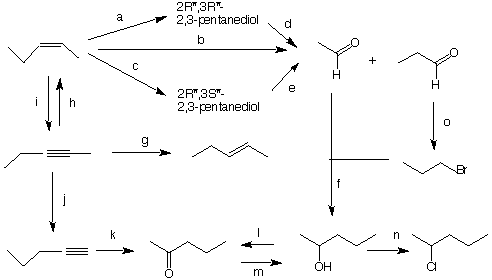
ways. C forms G (C3H6O2), E forms H (C2H4O2), and F is inert. What are the structures of A-H? Explain and illustrate. 5. Provide the structures of A-E in the sequence of reactions shown below. Provide a brief explanation. The reaction of A ---> B is a two-electron reduction that is not in your text. Can you write a mechanism for this reaction? 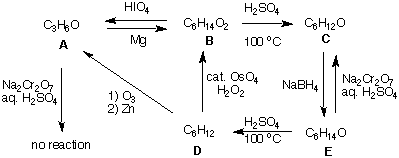 6. Provide reaction conditions for each of the the reagents a-o. In some instances, two steps will be required to complete the transformation. [There is more of this type of exercise on the Bulletinboard dated October 30.]
7. A graduate student believes that she has isolated (Z)-3-octen-1-ol A from a plant source. To confirm her hypothesis, she designs a synthesis of A using acetylene and ethylene as her only sources of carbon. All reagents are available to her. How might she have accomplished her synthesis? 8. Provide reaction conditions to accomplish the following chemical transformations. All necessary carbon sources and reagents are available to you.
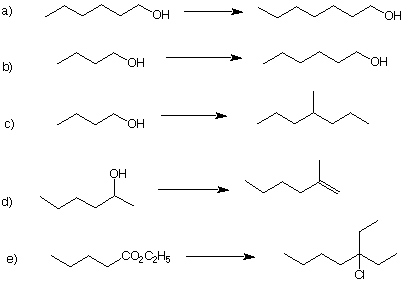 9. (R)-2-Butanol (A) reacts with SOCl2 in dioxane to produce (R)-2-chlorobutane (B). On the other hand, when A is converted into its tosylate C, the tosylate produces (S)-B upon reaction with LiCl. When either (R)-B or (S)-B is converted into its respective Grignard reagent and is subsequently reacted with acetaldehyde, the same mixture of diastereomeric alcohols C and D is produced, both of which are optically inactive. Alcohol C is formed via hydroboration and alkaline hydrogen peroxide oxidation of (E)-3-methyl-2-pentene (E), whereas alcohol D arises from the same treatment of (Z)-3-methyl-2-pentene (F). Provide structures for A-F and explain what is occurring. 10. How many grams of potassium dichromate (K2Cr2O7) are required to oxidize 0.1 moles of 3-pentanol in aqueous H2SO4? You may need to refresh your memory on how to balance redox reactions. |