Chem 220a
Problem Set 3
Chapter 4
Due: Monday, October 2, 2000

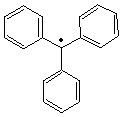
Moses Gomberg (1866-1947)
|
One
hundred years ago, Moses Gomberg (University of
Michigan) published his landmark paper, An Instance of
Trivalent Carbon: Triphenylmethyl. For the the first time, a
stable free radical had been characterized.
|
1) Study the alkane module in Organic
Reactions Go Online (ORGO).
2) Determine the heat of reaction for the propagation steps and
the overall reaction for the chlorination of neopentane
(2,2-dimethylpropane). Use the bond dissociation energies )BDEs) on
pg. 142 of your next or the ones at http://classes.yale.edu/chem220a/studyaids/thermo/BDE.html.
3) Benzene (1), and not toluene (2), is a suitable
solvent for the free radical bromination of cyclopentene (3).
Explain using BDEs.
4) Write a free radical chain reaction for the formation of the
major monobromination product in problem 2. Calculate the heat,
liberated or gained, for the propagation steps and overall reaction
in the formation of this substance. Show work.
5) Draw all the constitutionally isomeric monochlorination
compounds formed in the chlorination of 2-methylbutane. How much of
each one is formed? Show work.
6) Compare the free radical bromination and iodination of ethane
using chain reactions and BDEs. Create an energy diagram and label
the endothermic and exothermic steps and the thermicity for the
overall reaction. Be sure your diagram is in accord with the Hammond
Postulate. What are your conclusions.
7) Why is free radical chlorination of unsubstituted cycloalkanes
a suitable reaction for the formation of monochlorination products,
while the same process is unsuitable for the monochlorination on
n-alkanes.
a
