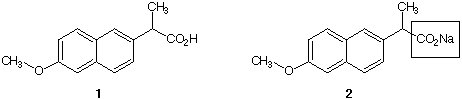Chem 220a
Problem Set 1
Chapters 1 and 2
Due: Monday, September 18, 2000

|
Antoine
Lavoisier (1743-1794)
French scientist who developed dual names for chemical
compounds. His work in chemical analysis led to the
overthrow of the phlogiston
theory (Stahl) by correctly explaining the role of
oxygen in combustion and the formulation of the law of the
conservation of mass.
|

|
Jons
Jacob Berzelius (1779-1848)
Swedish chemist who gave us the modern symbols of the
elements and discovered many of them. He was a proponent of
the dualistic (electrochemical) theory and he recognized and
defined the property of isomerism.
-
|
1) The analgesic Naproxen®
(1) is sold by prescription. Its sodium salt 2 is the
active ingredient in
Aleve®,
which is an over-the-counter analgesic. Draw resonance structures
for the carboxylate portion [in the box] of 2
using Lewis structures and line-angle formulas. You may use "R"
for the non-carboxylate part of the
structure.
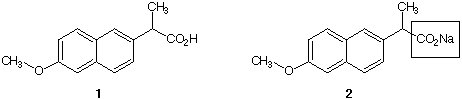
2) Draw line-angle formulas for the eight structural isomers of
C4H11N. These isomers are of the "amine
type"
(derivatives of ammonia).
3) For the following pairs of acids and bases, predict and explain
the equilibrium constant for the reaction: a)
methanol + NaNH2, b) CH3CO2H +
KCN, c) acetylene + KOH [http://classes.yale.edu/chem220a/
studyaids/pKa.html]
4) A sample (118 mg) of a hydrocarbon occupies a volume of 100 mL
at 27 oC and 740 mm pressure. What is
the structure of the hydrocarbon? Show work. [Hint: PV =
nRT]
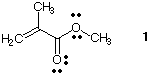
5) The polymer Plexiglas® is
formed by the polymerization of the monomer, methyl methacrylate
(1). Draw an
orbital representation of the monomer.
|
6) In a historical paper in 1852, Williamson reported the
preparation of an unsymmetrical ether. This new ether had a
vapor density approximately twice that of air. A combustion
analysis was conducted.
"... the ether being burnt by oxide of copper, the
following results were obtained:
0.2215 grm. of liquid gave:
0.482 " " carbonic acid [CO2], and
0.2685 " " water ..."
From these data, determine the molecular formula of this
ether and draw its structure. Show your work. [Remember:
Oxygen is not determined directly in a combustion analysis
but rather by difference. The ideal gas law relates
molecular weight and gas density at the same temperature and
pressure.]
|

Alexander
Williamson
(1824-1904)
|
7) Which of the following compounds have a net dipole moment?
Which ones do not? Explain.
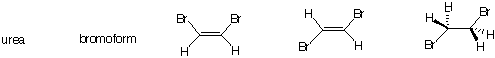
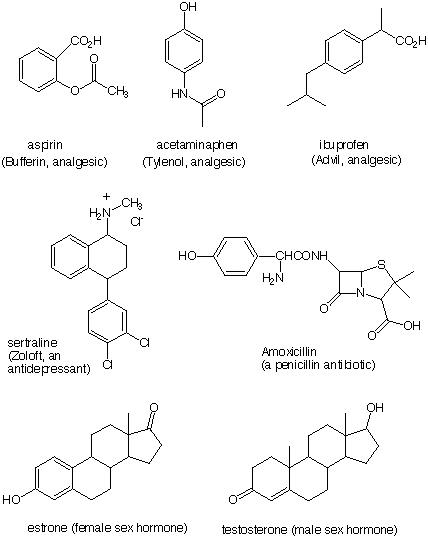
8) Locate the following functional groups in the compounds shown
below. Circle all the groups and place the appropriate letter next to
it. Some groups appear more than once; others are absent: a) thiol,
b) ketone, c) phenol, d) alcohol, e) aromatic ring not covered by
(c), f) alkene, g) amide, h) amine, i) ester, j) carboxylic acid and
k) aldehyde. The inside front cover of your text will be of
assistance.
SKYLAR: Well, have you studied organic chemistry?
WILL: A little bit.
SKYLAR: Oh, just for fun.
WILL: Yeah, for kicks.
SKYLAR: Yeah, it's SO much fun studying organic chemistry. Are you
mad? Have you completely lost your
mind? Nobody studies it for fun. It's not a necessity, especially
for someone like you. .....Good Will Hunting, Miramax Films