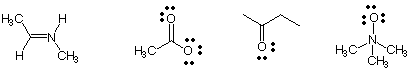
SKYLAR: Well, have you studied organic chemistry?
WILL: A little bit.
SKYLAR: Oh, just for fun.
WILL: Yeah, for kicks.
SKYLAR: Yeah, it's SO much fun studying organic chemistry. Are you mad? Have you completely lost your
mind? Nobody studies it for fun. It's not a necessity, especially for
someone like you. .....Good Will Hunting, Miramax Films
1) Draw Lewis structures and a line-angle formula for nitroethane (CH3CH2NO2). Use curved arrows to
illustrate resonance.
2) Draw charges on the following structures where they are needed. Justify your answers.
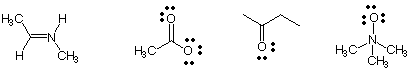
3) A pKa problem. Aqueous solutions of alkyllithiums (RLi, e.g.; CH3Li) reagents cannot be prepared.
Explain. What is formed?
4) A compound of MW = 72 showed the following composition: C = 66.63%; H = 11.09%. What is the
molecular formula of the compound? Show work.
5) Which compound is more polar, bromoform or carbon tetrabromide? Explain and illustrate.
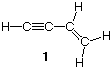
6) Vinylacetylene, which is prepared from acetylene and serves as an intermediate in the preparation of neoprene rubber, is represented by structure 1. Draw an orbital representation of vinylacetylene.
7) In what direction does the following equilibrium lie? Justify your answer.
![]()
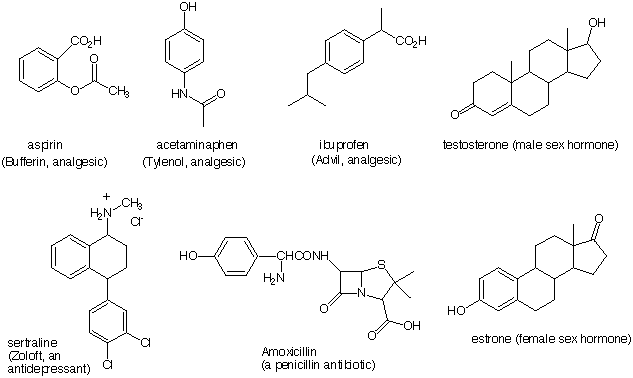
8) Locate the following functional groups in the compounds shown below. Circle all the groups and place the
appropriate letter next to it. Some groups appear more than once; others are absent: a) thiol, b) ketone, c)
phenol, d) alcohol, e) aromatic ring not covered by (c), f) alkene, g) amide, h) amine, i) ester, j) carboxylic
acid and k) aldehyde. The inside front cover of your text will be of assistance.