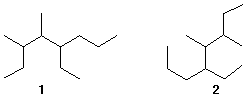
Explore the Conformation module in Study Aids. [http://www.yale.edu/chem220]
1) Baeyer [http://www.yale.edu/chem220/gallery/baeyer.html] did not believe Sachse's hypothesis that
cyclohexane has a chair conformation. What was Baeyer's argument against the Sachse hypothesis/?
2) Name the following alkanes.
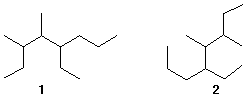
3) [SKYLAR: ... I can't right now. I've got to assign the proton spectrum for ibogamine (1). GWH, Miramax Films] We'll learn about proton spectra late in the term but for now, locate a cyclohexane ring in ibogamine. Why does the boat conformation of cyclohexane exist in ibogamine and yet it is unstable in cyclohexane itself?

4) Compute the percentage of equatorial and axial chlorocyclohexane at 27 oC. Use data from pg. 118. DGo = -RT ln Keq
5) Examine the heats of formation for the Alkanes in the Heats of Formation table (Study Aids). By what
average value (kcal/mol) does the heat of formation change when the chain length of an n-alkane is increased by a -CH2- group? Using this information and the heat of formation of cyclopropane, calculate the strain energy in cyclopropane. Use a similar method of calculation to the one presented on pg. 108. How well does your answer compare to the value obtained via heat of combustion?
6) There are three gauche butane interactions in cis-decalin and none in trans-decalin. Draw cis- and trans-
decalin. Locate these three (not four) interactions. Estimate the difference in energy between these stereoisomers using the value for -CH3 (pg. 118).
7) Draw the staggered and eclipsed conformations of 2-methylbutane about the C2-C3 bond. What is the energy of each conformation?