Due: Monday, March 25, 2013
Solution Set
In 1840, Christian Friedrich Schönbein (1799-1868) discovered ozone (Gr.; odorant), the sharp odor produced by electrical discharges. Seven years later (1847) he observed that ozone oxidizes organic compounds but not to their ultimate products of oxidation, carbon dioxide and water. [Two years prior, he had spilled nitric and sulfuric acid on his Frau's apron in her kitchen. The apron, made of cotton, combusted and thus was discovered gun cotton, nitrocellulose. Schönbein also observed that hydrogen peroxide (Threnard; 1818) is oxidized to oxygen gas in the presence of hemoglobin. ] In the period 1903-1916, Carl Dietrich Harries (1866-1923), an assistant to both Hofmann (of the eponymous elimination and rearrangement) and Fischer (of projection and carbohydrate fame) at Berlin, published some 80 papers on the reactions of ozone with organic compounds. His interest was stimulated by the reaction of ozone with rubber, a process that causes rubber to become hard and brittle. These studies led to the analytical and synthetic uses of ozone. From 1904-1916 he was a professor at Kiel. Disenchanted with academic life, he became Director of Research for Siemens and Halske, the German company co-founded by the electrical pioneer, Werner von Siemens, his father-in-law. Not surprisingly, Siemens went into the business of producing ozone generators. The studies of Rudolf Criegee (1902-1975; Karlsruhe) produced a unified mechanism for the process of ozonolysis. |

Carl Dietrich Harries (1866-1923) |
Reading assignments:
a)The alkene module in ORGO.b) Ozonolysis module.
How do I approach solving problems like 3 and 4? Here is a step-by-step analysis of a typical problem.
|
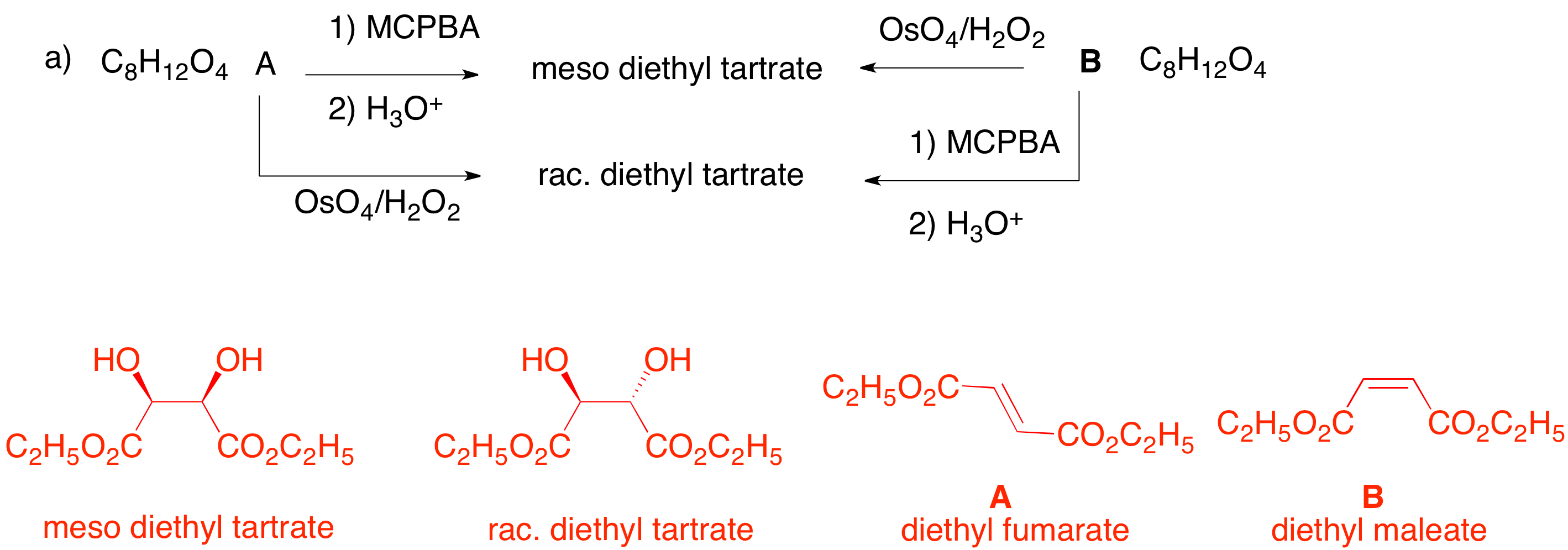
1. Provide the missing information in each of the following problems: reagents or unknown structures. Explain your reasoning . a) The reaction conditions are those for forming 1,2-diols from alkenes. OsO4 forms 1,2-diols by syn addition. Therefore, B must be the (Z)-diester, diethyl maleate. Epoxidation of B provides a meso-epoxide which undergoes anti opening in aqueous acid to yield racemic diethyl tartrate. Since the reaction conditions are reversed for reactions of A, it must be the (E)-diester, diethyl fumarate.
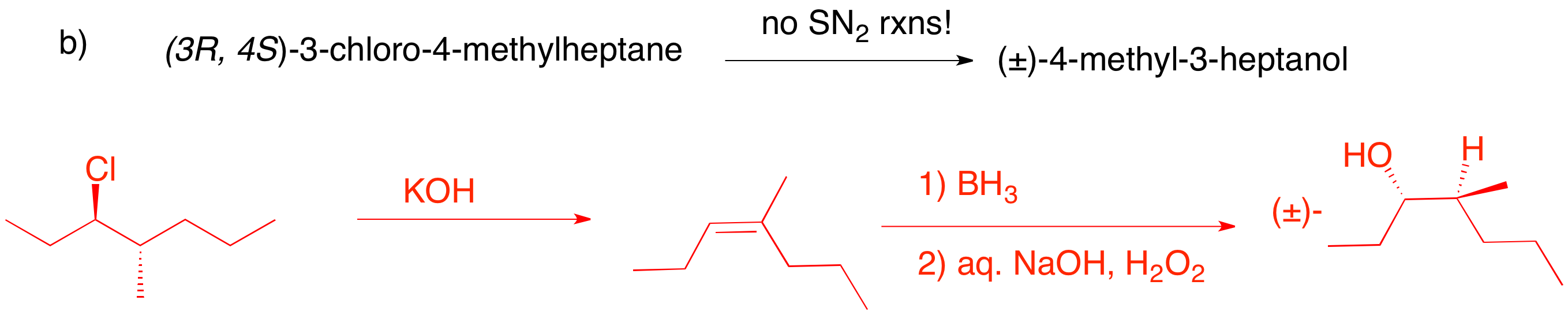
b) A small base will effect E2 elimination of HCl to give the more substituted alkene. Anti elimination of HCl gives the achiral (Z)-alkene. Hydroboration affords anti-Markovnikov addition of water to the alkene. Since both faces of the alkene are equally accessible leading to the racemic alcohol. Lesson: remove HCl anti; add water syn.
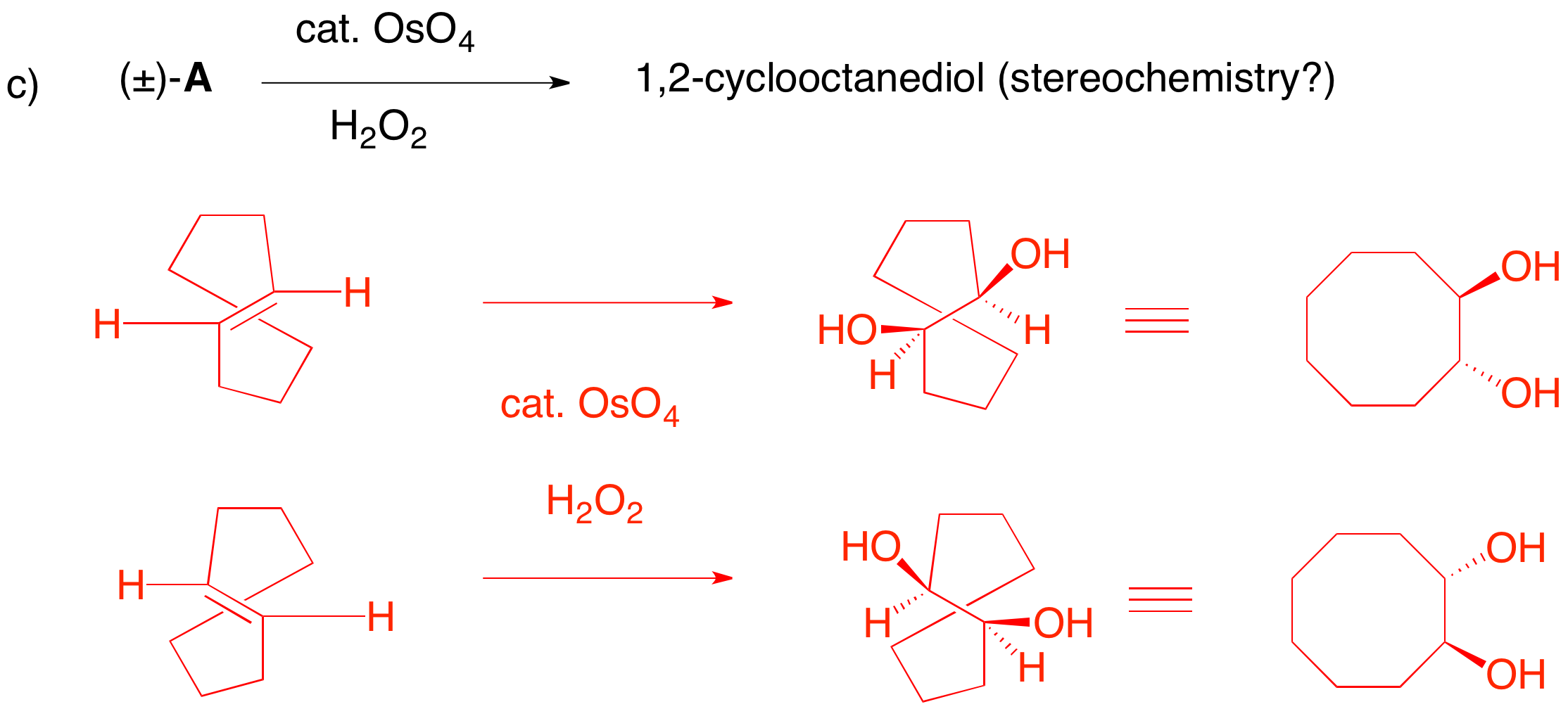
c) Racemic A has 2 degrees of unsaturation. Since one unsaturation unit is a cyclooctane ring, the other must be an alkene. Cis-cyclooctene is achiral but the trans-isomer can be a racemate. 1,2-Syn addition of the two hydroxyl groups can only occur on one face of each enantiomer because the underside of the double bond is protected by the ring methylene groups. In a "flat" structure of cyclooctane, this translates to a trans-diol.
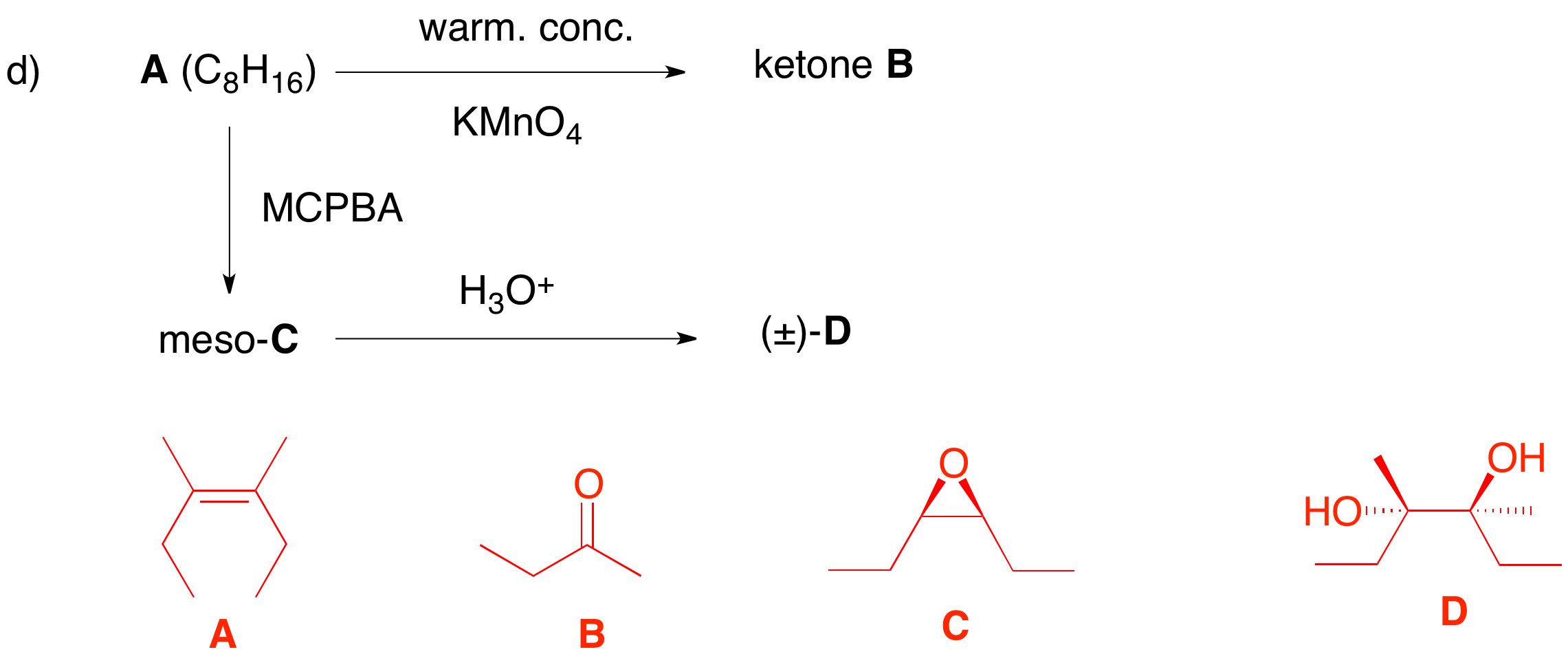
d) Structure A has one degree of unsaturation. From its reactions, it must be an alkene. The double bond is cleaved by permanganate, a la ozone, to give a single ketone. The ketone has to be C4 (2 x 4 = 8) and can only be B, 2-butanone. Peracid epoxidation is a syn addition and leads to a meso epoxide C. Therefore, A must be a (Z)-alkene. Aqueous acid opens the epoxide to provide racemic diol D. |
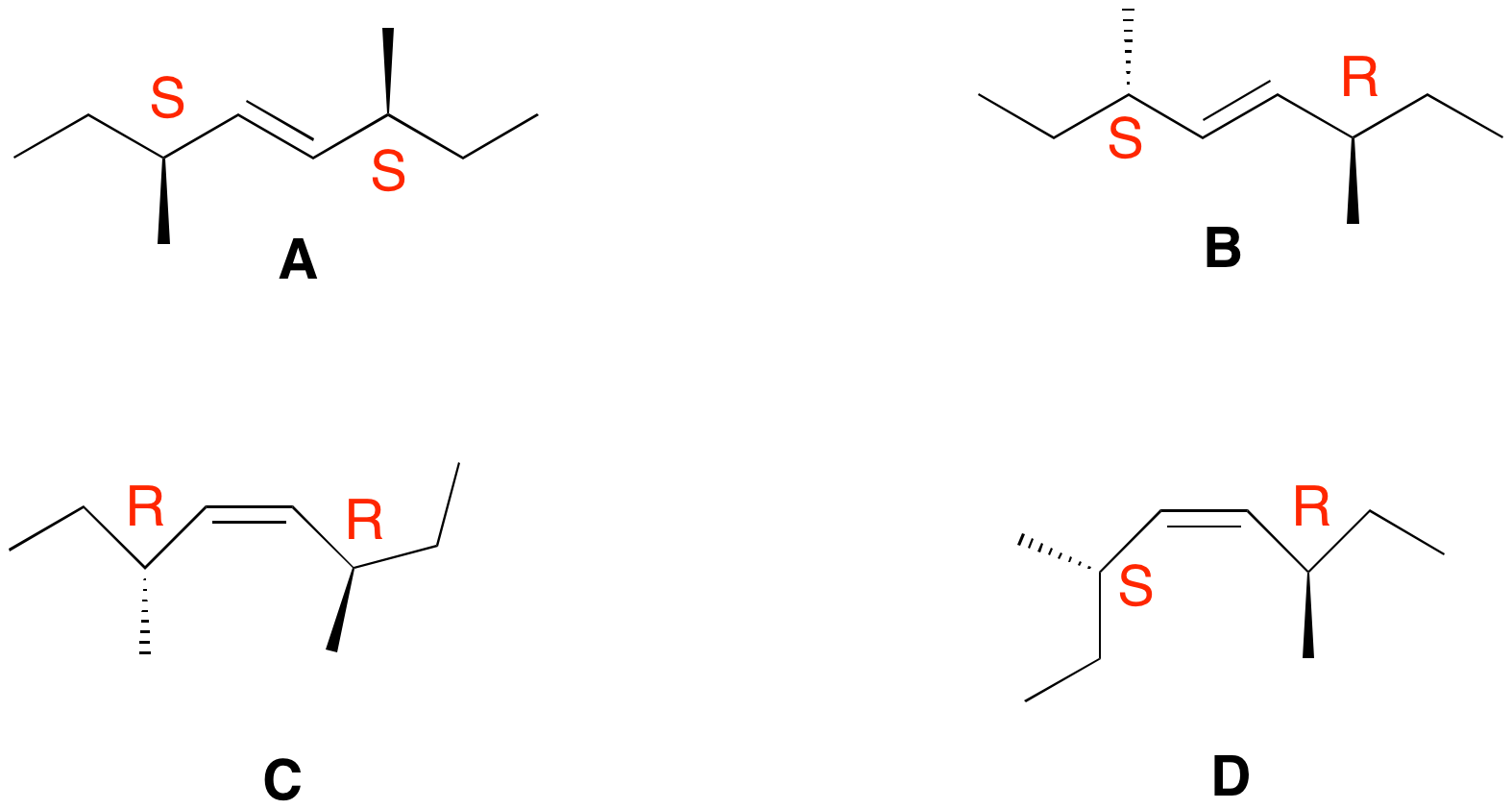
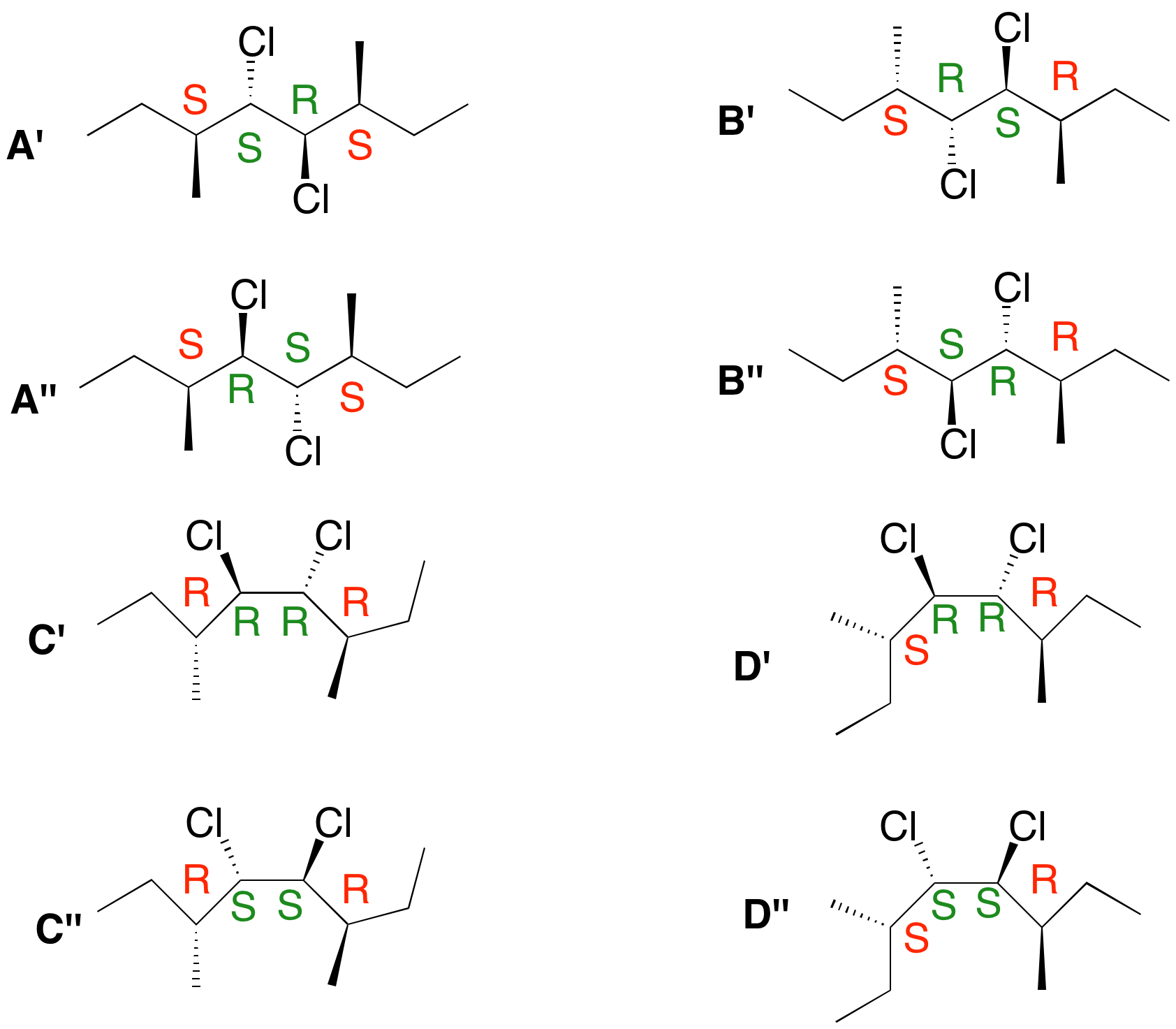
2. Consider the four alkene stereoisomers A-D shown on the right. a) Which ones are optically active? Which ones are not? Explain. The R,S descriptors are assigned to each structure on the right. Alkenes (S,S)-A and (R,R)-C are optically active. Alkenes (R,S)-B and (R,S)-D are not. In the case of D, rotation about the C3-C4 or C5-C6 bond will produce a conformation having a plane of symmetry. Not so for B, which has a center of symmetry at the center of the double bond. This case is precisely the same as the anti-conformation of meso tartaric acid and the figure eight knot! b) What is the product of ozonolysis of each stereoisomer? Be specific! To what degree are the alkenes distinguishable from one another as a result of the ozonolysis? Alkene A gives (S)-2-methylbutanal; C gives (R)-2-methylbutanal. The sign of optical rotation is in question. Alkenes B and D form racemic 2-methylbutanal. The following facts are true for A-D upon reaction with chlorine. One alkene affords a racemic dichloride. One other alkene forms a single optically-active dichloride. A third alkene forms two optically active dichlorides while the fourth alkene provides two optically inactive dichlorides. c) Which alkene forms which dichloride? Explain. [Hint: It is not necessary to draw the structures out. You can use R,S-notation for each of the four stereocenters in the dichlorides derived from A-D.] Consider (E)-alkene A. Simplify it to (E)-2-butene. Anti addition of chlorine gives
|
meso-2,3-dichlorobutane, which must be 2R,3S. In principle, there are two modes of anti-addition to A, giving A ' (SSRS) and A'' (SRSS reading left to right. In this case SSRS = SRSS. A' = A''. There is no plane or center of symmetry. Alkene A affords a single optically active dichloride. Dichlorides D' and D" form a racemate, which of course, is optically inactive. B' and B'' are two optically inactive dichlorides. Note that SR|SR and SS|RR have a plane of symmetry. Finally, dichlorides C' and C'' are optically active stereoisomeric dichlorides. Knowing the mechanism of chlorination, all four alkenes are distinguishable in this experiment! |
|
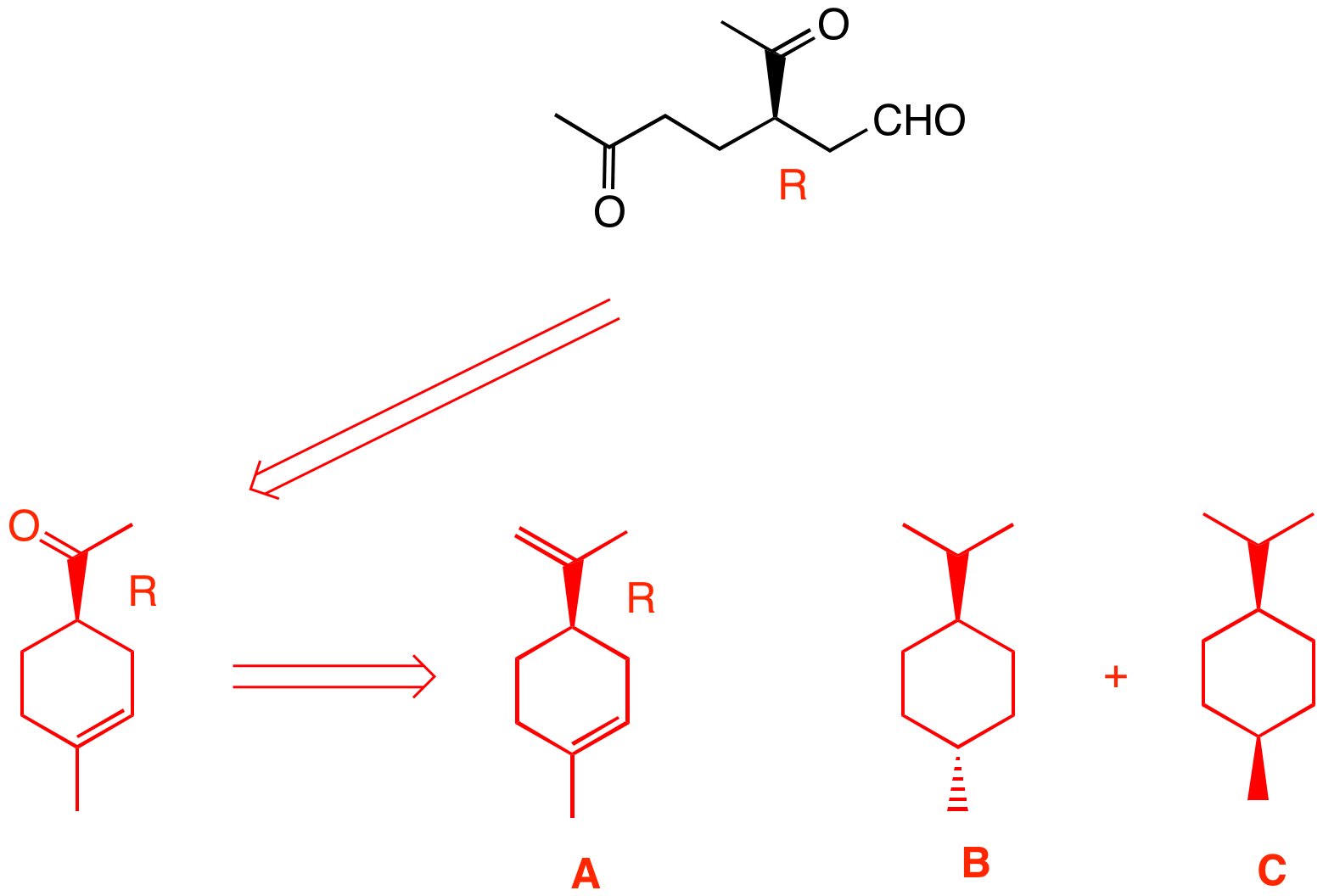
3. Optically active monoterpene A reacts with 2 molar equivalents of hydrogen to produce diastereomeric, disubstituted cyclohexanes B and C, both of which are optically inactive. Compound B has a smaller heat of combustion than C. Ozonolysis and dimethyl sulfide reduction of A affords the compound on the right as a reaction product as its (R)-enantiomer [Hint: Count carbons]. What are the structures A-C? Explain and illustrate. A monoterpene is a C10 compound, a natural product. A absorbs two moles of hydrogen (2 D.U.) and leads to two cyclohexanes (1 D.U.). Thus, A has 3 D.U. and its formula is C10H16. [Double 3, subtract from 22]. There is only one way to form a six-membered ring from the ozonolysis product. Connect the aldehyde carbon with the remote ketone carbon. The product of ozonolysis has 9 carbons, one C1 carbonyl compound is missing, formaldehyde, CH2=O. Formaldehyde and the remaining methyl ketone group lead to the structure for A. The hydrogenation gives trans B and cis C. B emits less heat on combustion than C because it is more stable (trans). If you had read StudyAids/Mechanism/Ozonolysis, you would have seen this problem at the end as well as the "Try this problem" link. |
|
|
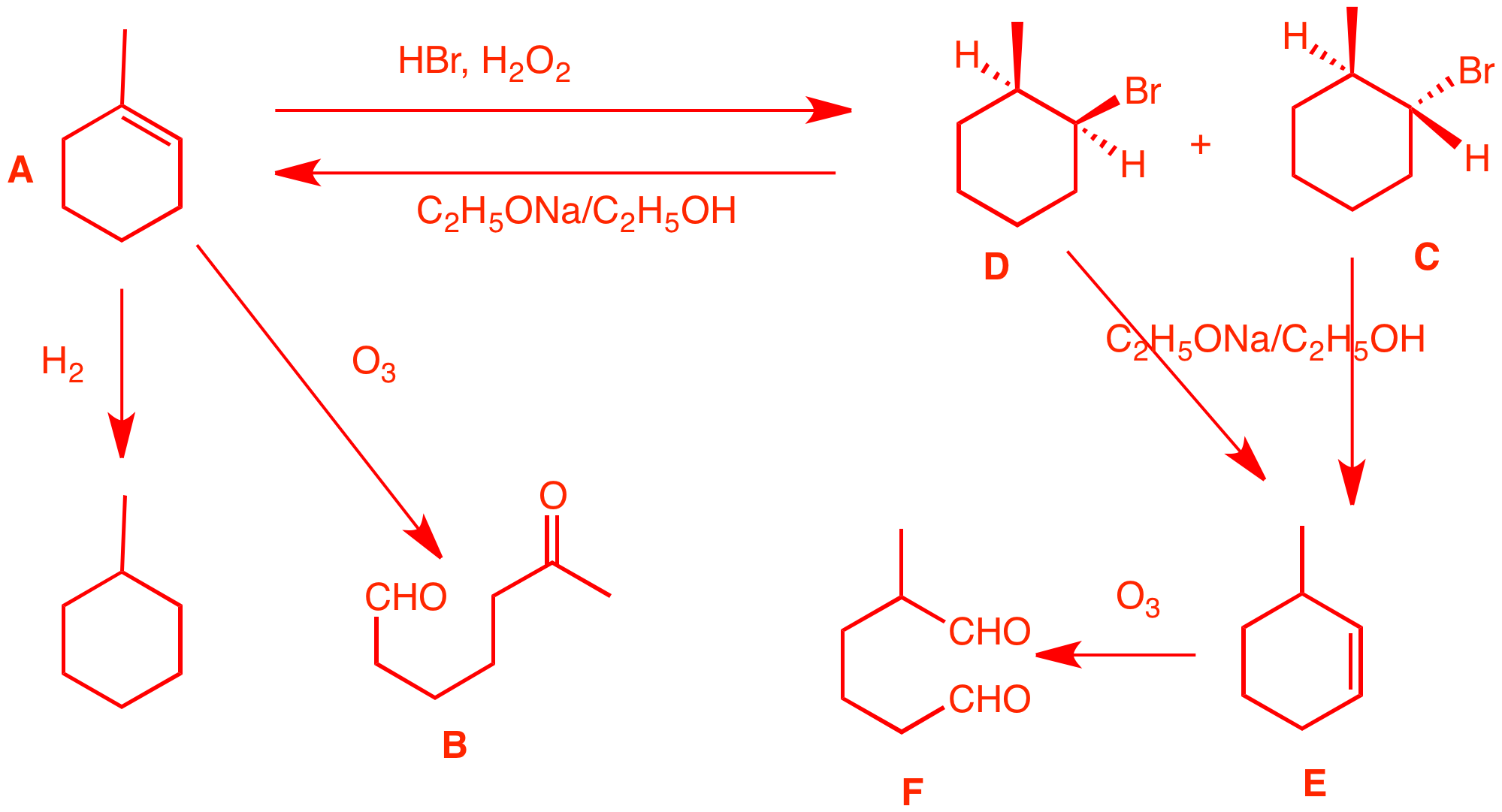
4. Compound A,
C7H12, [Degree
of Unsaturation?] affords
a
single
ketoaldehyde B upon ozonolysis and dimethyl
sulfide reduction. Hydrogenation of A gives
methylcyclohexane. Treatment of A with HBr in the
presence of
peroxide
gives two stereoisomeric bromides, C and D.
Compound C reacts with
C2H5ONa/C2H5OH
to give E while under the same conditions,
compound D gives mainly A and some of
compound E. Ozonolysis of E gives a single
dialdehyde F. What are the structures of
A-F? Explain and illustrate. Pay attention to
stereochemistry. DU = 2. The reaction
with O3 suggests an alkene and
hydrogenation gives methylcyclohexane. The
double bond must be trisubstituted because there
is a single keto
aldehyde formed. A must be
1-methyl-1-cyclohexene. Peroxide and HBr
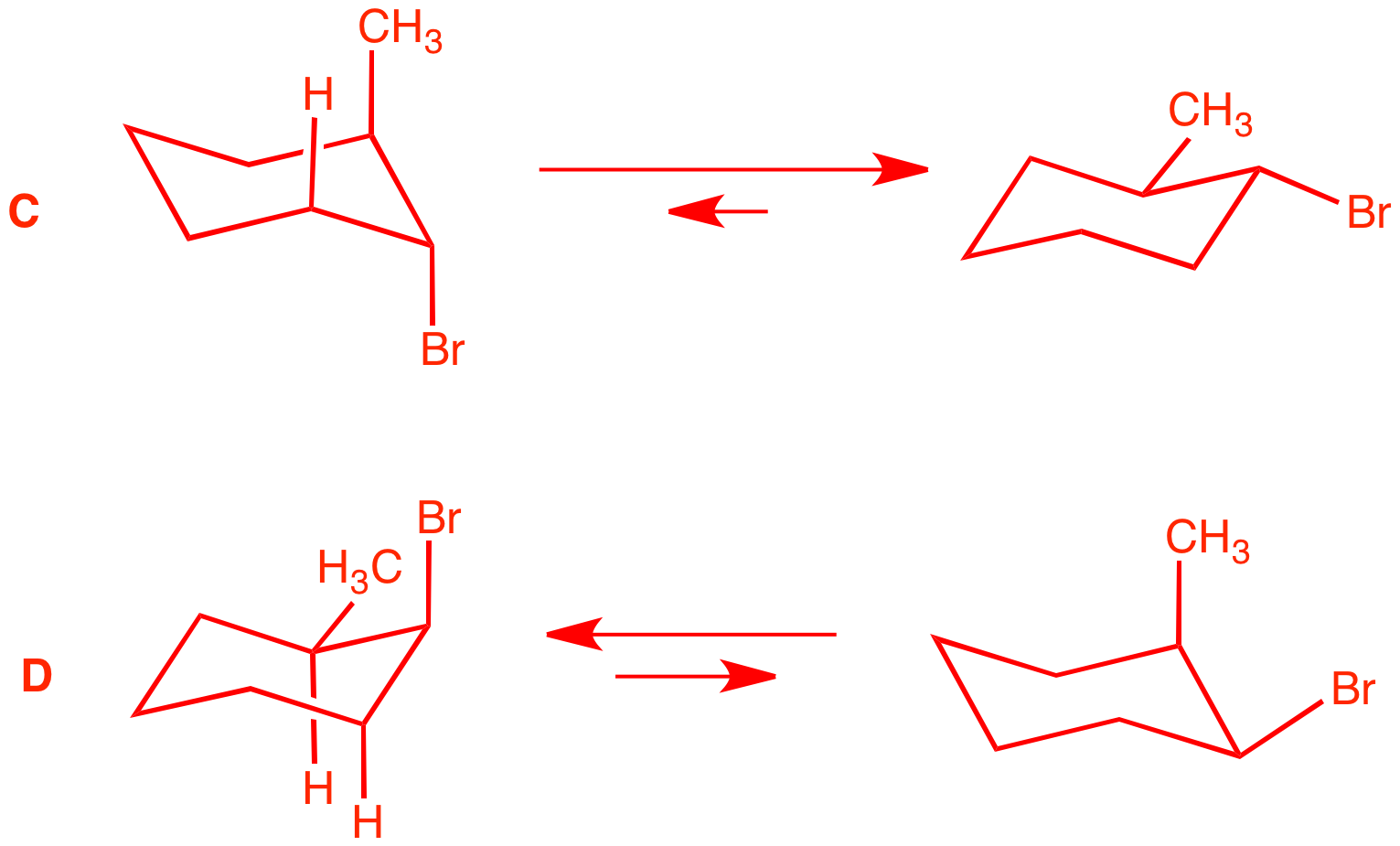
produces bromine radicals that add to the less
substituted end of the double bond of A. Stereochemistry is
established in the second propagation
step. C and D are cis and trans
2-methyl-1-bromocyclohexane. But which one is
which? The base treatment to give E2 elimination gives the answer.
|