,
Problem Set 6
Chapter 7, Structure and Synthesis of Alkenes
Due: Monday, March 4, 2013
Solution Set
|
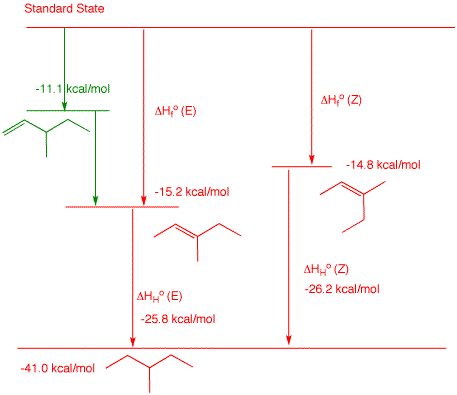
2. a) Determine the heat of formation of 3-methyl-1-pentene by using the heat of formation tables to determine typical heats of hydrogenation for monosubstituted alkenes. Show work. The hydrogenation product of 3-methyl-1-pentene is 3-methylpentane, ΔHfo = -41.0 kcal/mol. Looking at the ΔHfo of typical 1-alkenes, one can see: 1-butene (ΔHfo = 0 kcal/mol), 1-pentene (ΔHfo = -5.0 kcal/mol) and 1-hexene (ΔHfo = -10.2 kcal/mol). Notice each one differs by ~5 kcal/mol. Their respective alkanes are: n-butane (ΔHfo = -30.0 kcal/mol), n-pentane (ΔHfo = -35.1 kcal/mol) and 1-hexene (ΔHfo = -39.9 kcal/mol). The heats of hydrogenation of each of these is, respectively, the difference in the heats of formation: -30.0 kcal/mol, -30.1 kcal/mol and -29.7 kcal/mol. The average is -29.9 kcal/mol. Therefore, the ΔHfo of 3-methyl-1-pentene is x = -41.0 -(-29.9) = -11.1 kcal/mol. The reported ΔHfo of 2-methyl-1-butene is -6.1 kcal/mol. Add a methylene to this structure (-5.0 kcal/mol) and you obtain ΔHfo = -11.1 kcal/mol for 3-methyl-1-pentene. b) Calculate the heat of hydrogenation of (E)-and (Z)-3-methyl-2-pentene. Show work. From the Heats of Formation Table, (E)-3-methyl-2-pentene is ΔHfo = -15.2 kcal/mol and (Z)-3-methyl-2-pentene is ΔHfo = -14.8 kcal/mol. The heat of formation of 3-methylpentane is ΔHfo = -41.0 kcal/mol. The heat of hydrogenation of the (E)-isomer is -41.0 - (-15.2) = -25.8 kcal/mol. The heat of hydrogenation of the (Z)-isomer is -41.0 - (-14.8) = -26.2 kcal/mol.
|
|---|
|
3. The major product B from free radical monobromination of alkane A (C6H14) readily reacts with water to form C, C6H14O. Treatment of bromide B with KOH in ethanol produces two, and only two, compounds, D and E. Compound D liberates 1.6 kcal/mol more heat upon hydrogenation to A than does E. What are the structures A-E? What is the heat of isomerization of D to E? Illustrate and explain. [Hint: What are the structures possible for A?] |
| There are five possible structures, 1-5, for the acyclic, saturated alkane A. Since bromide B reacts readily with water (SN1) to form alcohol C, structures 1 and 5 are eliminated because they do not have any tertiary hydrogens. Structure 3 is also eliminated because E2 elimination of 3-bromo-3-methylpentane could give three alkenes: 2-ethyl-1-butene and (E)- and (Z)-3-methyl-2-pentene. That leaves 6 and 7 from 2 and 8 and 9 from 4 for the structures of D and E. Lookup the heats of formation of 4, 8 and 9 in the Heats of Formation Tables. From these data, one calculates the difference in the heat of hydrogenation as 1.1 kcal/mol, which isn't the value 1.6 kcal/mol. Alternatively, Table 7-1 in Wade gives the difference as 1.4 kcal/mol. (there is some discrepancy here.) Neither 6 nor 7 appear in the heats of formation table but we can look at lower homologs and calculate the heats of formation using the 5.0 kcal/mol/CH2 rule. Thus, 2-methyl-1-butene (ΔHfo = -8.4 kcal/mol) gives ΔHfo = -13.4 kcal/mol for 7 while 2-methyl-2-butene (ΔHfo = -10.0 kcal/mol) affords ΔHfo = -15.0 kcal/mol for 6. The difference is 1.6 kcal/mol. [There is actually no need to include the 5 kcal/mol correction because the difference would be the same.] Using Table 7-1 in Wade, a value of 1.6 kcal/mol is obtained. So, A = 2-methylpentane; B = 2-bromo-2-methylpentane; C = 2-methyl-2-pentanol; D = 2-methyl-1-pentene (7); E = 2-methyl-2-pentene (6). D, with a less negative heat of formation than E, liberates more heat on hydrogenation. | 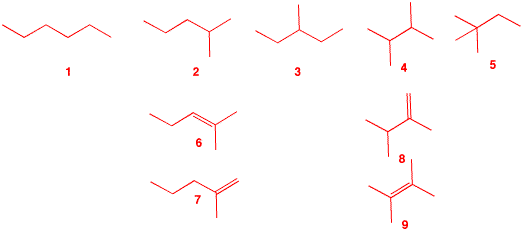 |
|---|
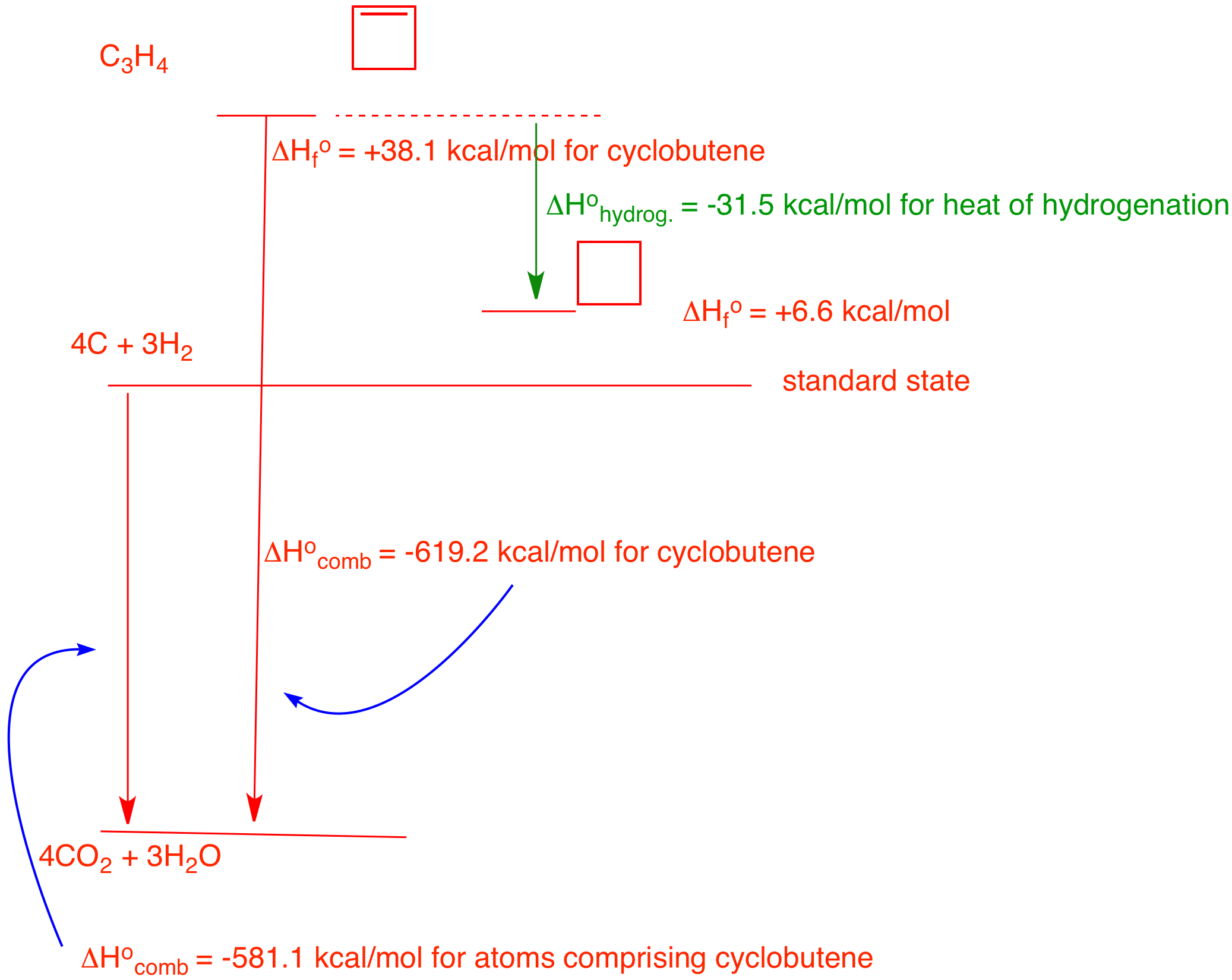
4. In 1968 Wiberg and Fenoglio determined the heat of combustion of cyclobutene: -619.2 kcal/mol. Determine the heat of hydrogenation of cyclobutene to cyclobutane. You will need to use the heat of formation tables. Explain and illustrate with a diagram.
K. B. Wiberg
|
You are given the heat of combustion of cyclobutene. The heat of formation of cyclobutene can be calculated by first determining the heat of combustion of 4 moles of graphite and 3 moles of hydrogen from the standard state. The Heat of Formation Tables gives the heat of formation of CO2 as (-94.05 kcal/mol) and water (-68.3 kcal/mol) at 25 oC for a total of -581.1 kcal/mol for 4C and 3H2 from the elements. Thus, the heat of formation of cyclobutene is the difference between these numbers (-581.1) - (-619.2) = +38.1 kcal/mol. Clearly, cyclobutene is less stable than the elements from which it is formed. The Tables give the heat of formation of cyclobutane. The heat of hydrogenation of cyclobutene to cyclobutane is (+6.6) - (+38.1)) = -31.5 kcal/mol.
|
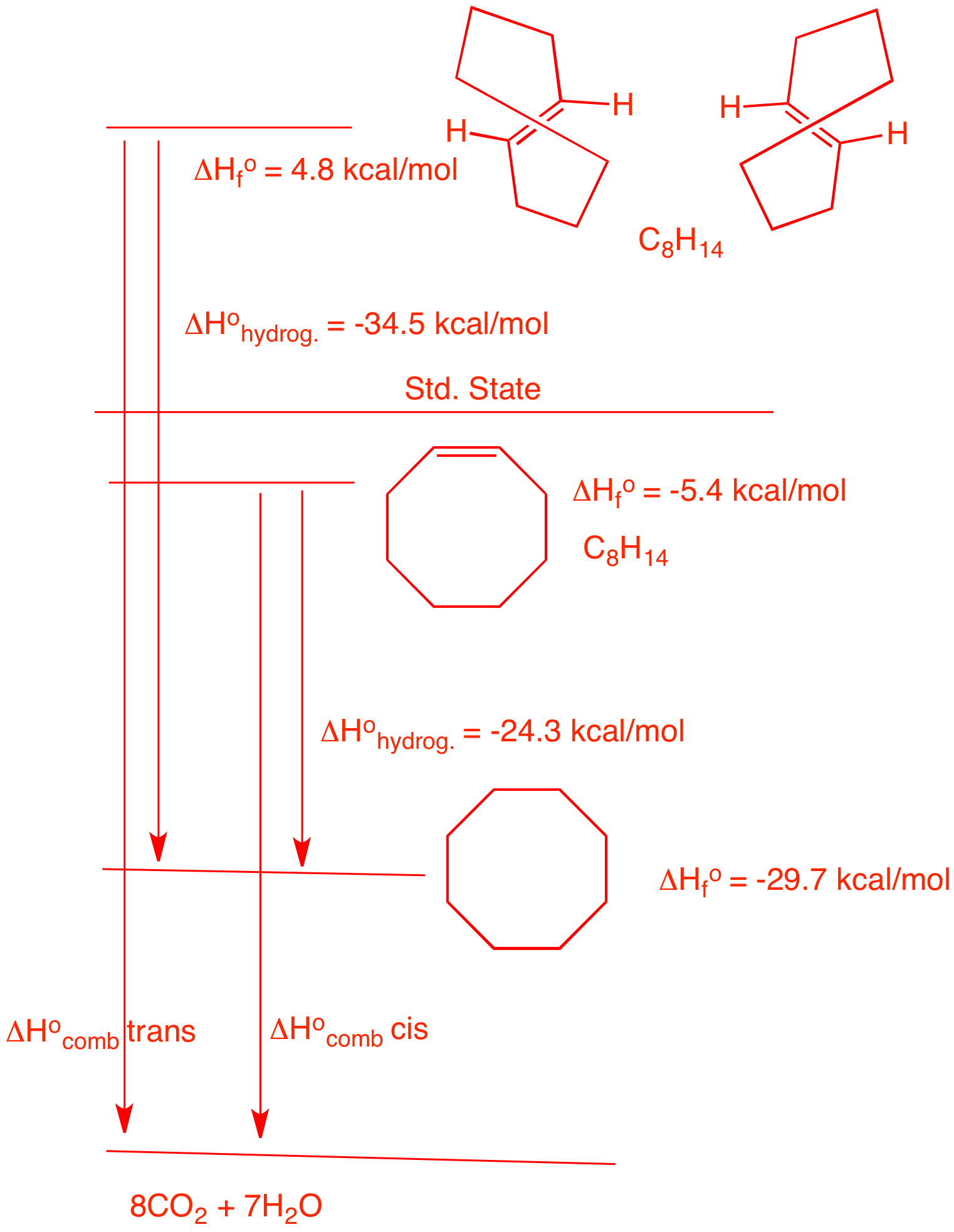
| 5. Two stereoisomers, A and B, absorb one equivalent of hydrogen upon catalytic
hydrogenation to form cyclooctane. Compound A, which
is capable of resolution, liberates 34.5 kcal/mol of heat
while B liberates 24.3 kcal/mol of heat. a) What are the structures of A and B ? Heat is liberated means that the reactions are exothermic. The numerical values are negative. Since A and B both absorb only one equivalent of hydrogen to form cyclooctane, they must be cis- and trans-cyclooctene. trans-Cyclooctene is more strained than cis-cyclooctene because a chain of six methylene groups is the minimum length to span a double bond that is trans. It gives off more heat on hydrogenation. Therefore, A is trans; B is cis. b) What are the heats of formation of A and B ? The heat of formation of cyclooctane is from the Heats of Formation Tables. The heats of hydrogenation are given. The differences gives the respective heats of formation. c) What is the difference in strain energy between A and B ? The difference between the heats of formation: 10.2 kcal/mol. d) What is the difference in the heat of combustion between A and B? From the diagram on the right it is obvious than the difference in the heats of combustion is the same as the difference in the strain energy of the two. e) Why is A capable of resolution? The non-superimposable mirror images of trans-cyclooctene do not interconvert. It is difficult for the vinyl hydrogens to pass through the ring to racemize. Link to JSmol structures here. |
 |