Problem Set 5
Chapter 6, Alkyl Halides: Substitution and Elimination
Due: Monday, February 25, 2013
Solution Set
Study #2 and #3 in the Alkyl Halide module and #1 in the Ether module in ORGO.
|
|
xxxxxxxxxxxxxxxxx |
Problem Set 5
Chapter 6, Alkyl Halides: Substitution and Elimination
Due: Monday, February 25, 2013
Solution Set
|
|
xxxxxxxxxxxxxxxxx |
|
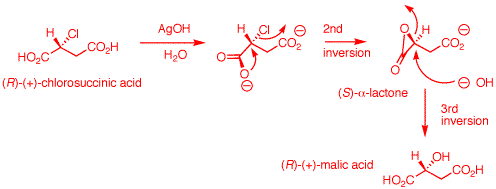
1. The inversion of configuration in an SN2 reaction is often called a Walden inversion, named after its discoverer, Paul Walden. In the cycle shown above, the overall conversion of one enantiomer of malic acid to the other one must require an inversion of configuration. Similarly, the same is true of the chloro acids. More generally, each interconversion of enantiomers must require an odd number of inversions. The PCl5 reaction requires a single inversion which means that the Ag2O reaction involves an even number of inversions of configuration, namely two in this instance. (-)-Malic acid is of the (S)-configuration. a) Show how malic acid, like any alcohol, might react with PCl5 and then undergo inversion to form a chloride. Remember that phosphoric acid is a strong acid and its conjugate base and analogs thereof are also good leaving groups. b) Silver oxide is an anhydrous form of AgOH. The carboxylic acid group closest to the hydroxyl group plays a role in the process. The reaction medium is mildly alkaline. Using these data, show how there is net retention of configuration. c) Draw these four enantiomers as Fischer projections
with the CO2H closest to the OH or Cl in the
topmost position. (-)-Malic acid is of the
(S)-configuration. |
b) (R)-Chlorosuccinic acid under alkaline conditions is converted to its dicarboxylate salt. Ag+1 may or may not complex with the chlorine atom at this point to enhance chloride as a leaving group. AgOH is not critical. The reaction works using NaHCO3 as a base. The proximate carboxylate acts as a nucleophile with SN2 inversion to form the reactive, transitory (S)-α-lactone. The strain of the α-lactone allows hydroxide to effect a second SN2 displacement to form (R)-malic acid upon acidification. This step has an even number of inversions -- net retention. The overall process of (R)- to (S)-malic acid has an odd number of inversions. |
|
c) 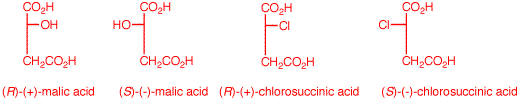 |
|---|
2. Predict the products in each of the following reactions. Provide a mechanism and rationale for each example.
a) Mercaptans (thiols) are
stronger acids than water. See pKa
table. Hydroxide ion converts
ethanethiol to its thiolate (mercaptide). The anion is a very good
nucleophile. Inversion of configuration occurs. Cl and S are both top
priority. Thus, (S)- becomes (R)-. |
|
3. Provide reaction conditions for each of the following chemical transformations. Each one will require several steps; show all steps leading to the final structure. Provide a rationale as to why each new compound is expected to be the major product.
a) The question is: How do I introduce a double bond into a specific position in an alkane given what I know thus far? Since you know how to introduce halogen into an alkane and you know how to effect E2 elimination, this is a useful point of departure. A tertiary halide would be useful since it can undergo Zaitsev elimination leading to the more substituted alkene when a small base is employed. But which halogenation should be employed? Chlorination or bromination. The selectivity for chlorination is 1o(1):2o(4.5):3o(5.5) [Wade’s Text], while bromination is approximately 1o(1):2o(82):3o(1600) [See slide 12 in Radical.ppt., Anson, Fredricks, Tedder (1958)]. Clearly, bromination will be more selective for a tertiary hydrogen (~83%) than chlorination (~17%). This computation is in PS3 #1 and in slide 9 of Radical.ppt. b) Tertiary halides are susceptible to solvolysis (SN 1) while primary halides are not! First treat the dichloride with an excess of ethanol to form the ethyl ether. Notice that no strong base (C2H5ONa) was used. This would cause substitution at the primary site (SN2) and E2 elimination at the teriary halide position [Cf. 4a]. Now use sodium methoxide/methanol to facilitate an SN2 substitution at the primary chloride. The existing ethyl ether is stable to these conditions. If this were not true, you couldn't prepare tertiary alkyl ethers by the Williamson ether synthesis. c) For the first step, see 4a. E2 elimination of HBr gives a single product, isobutylene. Peroxide-induced, anti-Markovnikov addition of HBr to the alkene affords isobutyl bromide (1-bromo-2-methylpropane). [If we haven't discussed "Markovnikov addition" at this point, look it up in your text.] d) Formation of the bromide follows the protocol in 4c. How to obtain the alkene from the chloride? Use a bulky base (potassium t-butoxide) to minimize Zaitsev and maximize Hofmann E2 elimination. e) Allylic C-H bonds have low Bond Dissociation Energies (BDEs). There are four, equivalent allylic C-H bonds in the starting material alkene. A resonance stabilized radical is formed which, in the second propagation step reaction with bromine, leads to the more substituted alkene - least substituted bromide. Some structural bromide may be formed (shown in green). Use the mixture in an SN1 solvolysis reaction with excess water, a reaction that proceeds through the resonance stabilized allylic cation. The role of NBS (N-bromosuccinimide) is to generate low concentrations of bromine, which favor radical reactions of bromine vs. addition to the double bond. The difference has to do with the kinetics of the two reactions. Radical bromination is first order in bromine; addition is second order in bromine. |
|
|---|