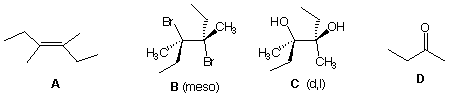
Problem:
Compound A, C8H16, forms a meso compound B upon reaction with bromine in CCl4 and a d,l-diol C upon oxidation with OsO4. Ozonolysis of A provides a single ketone D. What are the structures of A-D? Show how they are formed.
Solution:
1. Compound A, C8H16 : Compound A has one degree of unsaturation. C8H18 - C8H16 = 2, divided by 2 = 1. It is either a double bond or a ring.
2. meso compound B upon reaction with bromine in CCl4 and a d,l-diol C upon oxidation with OsO4 : If A reacts with Br2, A's one degree of unsaturation is a double bond, i.e., no ring. Secondly, from a meso compound B we can conclude that whatever is substituted at one end of the double bond must be the same as at the other end of the double bond. There are only 6 carbons available for substituents, 3 at each end. If one substituent is 3 carbons (n- or i-propyl), then the other is H. If one is ethyl, the other one is methyl. The double bond must be of the (E)-configuration because Br2 adds trans to double bonds and consequently a meso, and not d,l-compound from a ](Z)-olefin] is formed. Because OsO4 adds syn (cis) to a double bond, a d,l-diol C compound must be formed. The d,l-diol C does not tell us about the symmetry in A but the meso dibromide B does. Thus, every meso compound has a d,l-diastereomer but the converse is not necessarily true.
So what is A?
3. Ozonolysis of A provides a single ketone D. Ketone D must be 4 carbons. One carbon is for the C=O group which leaves 3 carbons, obviously an ethyl and methyl group. Thus A must be (E)-3,4-dimethyl-3-hexene; B is a single diastereomeric dibromide (3R, 4S)-3,4-dibromo-3,4-dimethylhexane [ or (3S, 4R)-3,4-dibromo-3,4-dimethylhexane]; C is also a single diastereomeric diol as a pair of enantiomers (3R, 3R)-3,4-dimethyl-3,4-hexanediol and (3R, 3R)-3,4-dimethyl-3,4-hexanediol; D is 2-butanone methyl ethyl ketone, a.k.a., MEK). If you wish to designate relative stereochemistry for an achiral or racemic compound it is customary to assign the lowest numbered center thr R configuration and then add a superscripted asterisk. Thus B is (3R*, 4S*)-3,4-dibromo-3,4-dimethylhexane and C is (3R*, 3R*)-3,4-dimethyl-3,4-hexanediol. In this highly symmetrical case, meso and d,l is sufficient. Quod Erat Demonstrandum.