|
1. Read Degree
(Elements) of Unsaturation. How
many degrees of unsaturation are present in
C10H12BrClN2OS? Draw two
structures, one cyclic, the other acyclic, that have the
number of degrees of unsaturation you determined and that is
necessarily in agreement with the formula. b) Calculate the heat of
hydrogenation of (E)-and
(Z)-3-methyl-2-pentene. Show work. |
Paul Sabatier 1912 Co-Nobel Prize in Chemistry Hydrogenation by Metal Catalysis
|
|
4. Compound A
(C5H11Br) reacts readily with water to
form B, C5H12O. Exposure of
compound A to aq. NaOH gives only C (major)
and D (minor). Hydrogenation of C liberates
26.8 ± 0.1 kcal/mol heat while D liberates 28.4
± 0.1 kcal/mol of heat during hydrogenation. Both
C and D form E upon hydrogenation. What
are the structures A-E? Explain. |
|
5. a) Determine the heat of hydrogenation
of cyclohexene from the heat
of formation tables. b) How does
this value compare with the heat of hydrogenation of an
unstrained cis-disubstituted double bond? c) Given the heat
of hydrogenation of cyclopentene (chapter 7) determine the
heat of formation of cyclopentene. d) BONUS: Why is the heat
of hydrogenation less for cyclopentene than that for
cyclohexene? Show all work. |
|
6. Two stereoisomers, A and
B, absorb one equivalent of hydrogen upon catalytic
hydrogenation to form cyclooctane. Compound A, which
is capable of resolution, liberates 34.5 kcal/mol of heat
while B liberates 24.3 kcal/mol of heat. |
|
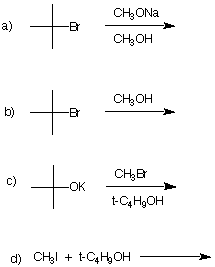
7. Comment critically on the following
proposed synthesis of the now banned gasoline additive,
methyl tertiary-butyl ether (MTBE). If you believe the
reaction will be successful, provide the type of mechanism
that is operable and illustrate it with the curved arrow
formalism. If you feel that the reaction will not be
successful, state the expected product of the reaction and
the mechanism by which it is formed. Illustrate with curved
arrows. If no reaction takes place, state so and explain why
not. |